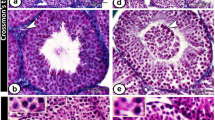
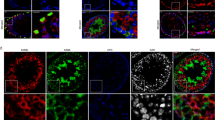
Abstract
Inhibin, a member of the TGF-β superfamily, is synthesized in the testis by Sertoli cells and exerts an endocrine regulatory function on pituitary hormone synthesis. A distinct local function has been proposed, negatively controlling cellular growth in the testis (tumor suppressor activity). A critical test for the identification of a tumor suppressor is the reversal of transformed growth properties upon re-expression of the gene in tumor-derived cell lines. Sertoli cell-derived tumoral lines were previously established from tumors that develop in elderly transgenic males which express in the testis the large T antigen of polyoma virus. Both the tumors and the cells in culture exhibited reduced levels of the inhibin α subunit mRNA. Stable transfectants were generated, in which this subunit was expressed from a heterologous promoter. All of them exhibited a strict inhibition of growth at confluency. On the other hand, in addition to an aging-related decrease in inhibin synthesis, the α subunit gene was down regulated in vivo in cells expressing the viral protein. The conjunction of these two factors accounts for the age-related occurrence of testicular cancers in the transgenic model, again pointing to inhibin as a potent cell growth regulator in the seminiferous epithelium.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Barut B, Chauhan D, Uchiyama H and Anderson KC. . 1993 J. Clin. Invest. 92: 2346–2352.
Boitani C, Stefanini M, Fragale A and Morena AR. . 1995 Endocrinology 136: 5438–5444.
Chen C-LC. . 1993 Endocrinology 132: 4–5.
Chen C-LC, Feng Z-M, Morris PL and Bardin CW . (1992). In: Molecular Biology of Reproduction, Serono Symposia. Spera G, Fabbrini A, Gnessi L and Bardin CW. (eds).. Raven Press: New York pp. 97–108.
Chomczynski P and Sacchi S. . 1987 Anal. Biochem. 162: 156–159.
Cowie A and Kamen R. . 1984 J. Virol. 52: 750–760.
DeLucia AL, Lewton BA, Tjian R and Tegtmeyer P. . 1983 J. Virol. 46: 143–150.
Dunbar CE, Browder TM, Abrams JS and Nienhuis AW. . 1989 Science 245: 1493–1496.
Dyson N, Bernards R, Friend SH, Gooding LR, Hassell JA, Major EO, Pipas JM, Vandyke T and Harlow E. . 1990 J. Virol. 64: 1353–1356.
Gaudray P, Tyndall C, Kamen R and Cuzin F. . 1981 Nucl. Acids Res. 9: 5697–5710.
Grandjean V, Vincent S, Martin L, Rassoulzadegan M and Cuzin F. . 1997 Biol. Reprod. 57: 1115–1122.
Grimaldi P, Geremia R, Albanesi C and Rossi P. . 1996 Mol. Cell. Endocrinol. 117: 167–173.
Guerra GR, Franco J and Gonzales GF. . 1989 Arch. Androl. 22: 35–40.
Hakovirta H, Kaipia A, Soder O and Parvinen M. . 1993 Endocrinology 133: 1664–1668.
Hangoc G, Carow CE, Schwall R, Mason AJ and Broxmeyer HE. . 1992 Exp. Hematol. 20: 1243–1246.
Huang HJ, Yee JK, Shew JY, Chen PL, Bookstein R, Friedmann T, Lee EY and Lee WH. . 1988 Science 242: 1563–1566.
Jakubowiak A, Janecki A, Tong D, Sanborn BM and Steinberger A. . 1991 Mol. Cell. Endocrinol. 82: 265–273.
Keating MT and Williams LT. . 1988 Science 239: 914–916.
Larsson S, Charlieu J, Miyagawa K, Engelkamp D, Rassoulzadegan M, Ross A, Cuzin F, van Heyningen V and Hastie N. . 1995 Cell 81: 391–401.
Matzuk MM, Finegold MJ, Su J-GJ, Hsueh AJW and Bradley A. . 1992 Nature 360: 313–319.
Pal D, Collins TJ and Parkening TA. . 1991 Biol. Reprod. 45: 869–875.
Paquis-Flucklinger V, Michiels J, Vidal F, Alquier C, Pointis G, Bourdon V, Cuzin F and Rassoulzadegan M. . 1993 Oncogene 8: 2087–2094.
Pech N, Hermine O and Goldwasser E. . 1993 Blood 82: 1502–1506.
Pelletier J, Schalling M, Buckler AJ, Rogers A, Haber DA and Housman D. . 1991 Genes Dev. 5: 1345–1356.
Rassoulzadegan M, Gaudray P, Canning M, Trejo-Avila L and Cuzin F. . 1981 Virology 114: 489–500.
Rich KA, Bardin CW, Gunsalus GL and Mather JP. . 1983 Endocrinology 113: 2284–2293.
Skinner MK. . 1993 The Sertoli Cell. Russell LD, Griswold MD (eds). Cache River Press: Clearwater FL pp. 237–247.
Su JG and Hsueh AJ. . 1992 Biochem. Biophys. Res. Commun. 186: 293–300.
Tjian R. . 1978 Cell 13: 165–179.
van Dissel-Emiliani FM, Grootenhuis AJ, de Jong FH and de Rooij DG. . 1989 Endocrinology 125: 1899–1903.
Vincent S, Marty L and Fort P. . 1993 Nucl. Acids Res. 21: 1498.
Vincent S, Segretain D, Nishikawa S, Nishikawa S, Sage J, Cuzin F and Rassoulzadegan M. . 1998 Development 125: 4585–4593.
Zhu Z, Veldman GM, Cowie A, Carr A, Schaffhausen B and Kamen R. . 1984 J. Virol. 51: 170–180.
Acknowledgements
We thank C Boitani for the generous gift of recombinant activin, F Ranc for help in cell culture experiments and L Martin for help in setting up the RNase protection assay. The expert technical assistance of M Cutajar and Y Fantéi is gratefully acknowledged. Recombinant human hormones were kindly provided by the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases. This work was supported by grants from Association pour la Recherche sur le Cancer (France).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lopez, P., Vidal, F., Rassoulzadegan, M. et al. A role of inhibin as a tumor suppressor in Sertoli cells: down-regulation upon aging and repression by a viral oncogene. Oncogene 18, 7303–7309 (1999). https://doi.org/10.1038/sj.onc.1203143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203143