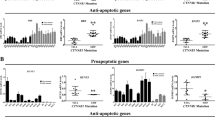
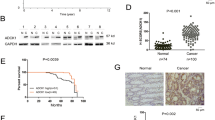
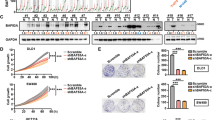
Abstract
The majority of human colorectal cancers have elevated β-catenin/TCF regulated transcription due to either inactivating mutations of the APC tumor suppressor gene or activating mutations of β-catenin. Surprisingly, one commonly used colorectal cancer cell line was found to have intact APC and β-catenin and no demonstrable β-catenin/TCF regulated transcription. However, this line did possess a truncating mutation in one allele of CDX2, a gene whose inactivation has recently been shown to cause colon tumorigenesis in mice. Expression of CDX2 was found to be induced by restoring expression of wild type APC in a colorectal cancer cell line. These findings raise the intriguing possibility that CDX2 contributes to APC's tumor suppressive effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aberle H, Bauer A, Stappert J, Kispert A and Kemler R. . 1997 EMBO J. 16: 3797–3804.
Brattain MG, Levine AE, Chakrabarty S, Yeoman LC, Willson JK and Long B. . 1984 Cancer Metastasis Rev 3: 177–191.
Chawengsaksophak K, James R, Hammond VE, Kontgen F and Beck F. . 1997 Nature 386: 84–87.
Ee HC, Erler T, Bhathal PS, Young GP and James RJ. . 1995 Am. J. Pathol. 147: 586–592.
Eshleman JR, Lang EZ, Bowerfind GK, Parsons R, Vogelstein B, Willson JK, Veigl ML, Sedwick WD and Markowitz SD. . 1995 Oncogene 10: 33–37.
Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt E, Lipkin M, Khan PM and Kucherlapati R. . 1994 Proc. Natl. Acad. Sci. USA 91: 8969–8973.
He T-C, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW. . 1998 Science 281: 1509–1512.
He TC, da Costa LT and Thiagalingam S. . 1997 Bioessays 19: 551–555.
Kinzler KW and Vogelstein B. . 1996 Cell 87: 159–170.
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B and Clevers H. . 1997 Science 275: 1784–1787.
Mallo GV, Rechreche H, Frigerio JM, Rocha D, Zweibaum A, Lacasa M, Jordan BR, Dusetti NJ, Dagorn JC and Iovanna JL. . 1997 Int. J. Cancer 74: 35–44.
Morin PJ, Vogelstein B and Kinzler KW. . 1996 Proc. Natl. Acad. Sci. USA 93: 7950–7954.
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B and Kinzler KW. . 1997 Science 275: 1787–1790.
Munemitsu S, Albert I, Souza B, Rubinfeld B and Polakis P. . 1995 Proc. Natl. Acad. Sci. USA 92: 3046–3050.
Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B and Kinzler KW. . 1992 Nature 359: 235–237.
Orford K, Crockett C, Jensen JP, Weissman AM and Byers SW. . 1997 J. Biol. Chem. 272: 24735–24738.
Riggins GJ, Markowitz S, Wilson JK, Vogelstein B and Kinzler KW. . 1995 Cancer Res. 55: 5184–5186.
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S and Polakis P. . 1993 Science 262: 1731–1734.
Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S and Polakis P. . 1996 Science 272: 1023–1025.
Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E and Polakis P. . 1997 Science 275: 1790–1792.
Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JK, Paraskeva C, Petersen GM, Hamilton SR, Vogelstein B and Kinzler KW. . 1993 Proc. Natl. Acad. Sci. USA 90: 2846–2850.
Sparks AB, Morin PJ, Vogelstein B and Kinzler KW. . 1998 Cancer Res. 98: 1130–1134.
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA and Dove WF. . 1992 Science 256: 668–670.
Su LK, Vogelstein B and Kinzler KW. . 1993 Science 262: 1734–1737.
Suh E, Chen L, Taylor J and Traber PG. . 1994 Mol. Cell. Biol. 14: 7340–7351.
Suh E and Traber PG. . 1996 Mol. Cell. Biol. 16: 619–625.
Wicking C, Simms LA, Evans T, Walsh M, Chawengsaksophak K, Beck F, Chenevix-Trench G, Young J, Jass J, Leggett B and Wainwright B. . 1998 Oncogene 17: 657–659.
Acknowledgements
This work was supported by NIH grants CA57345 and GM07814 and by a scholarship from the Fundação Luso-Americana para o Desenvolvimento (LT da Costa). B Vogelstein is an investigator of the Howard Hughes Medical Institutes. Genzyme Molecular Oncology (Genzyme) provided research support to KW Kinzler. B Vogelstein and KW Kinzler are consultants to Genzyme. The Johns Hopkins University and researchers (KW Kinzler and B Vogelstein) own Genzyme stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the University in accordance with its conflict of interest policies.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
da Costa, L., He, TC., Yu, J. et al. CDX2 is mutated in a colorectal cancer with normal APC/β-catenin signaling. Oncogene 18, 5010–5014 (1999). https://doi.org/10.1038/sj.onc.1202872
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202872
Keywords
This article is cited by
-
Heme oxygenase 1 protects human colonocytes against ROS formation, oxidative DNA damage and cytotoxicity induced by heme iron, but not inorganic iron
Cell Death & Disease (2020)
-
Role of mTORC1 in intestinal epithelial repair and tumorigenesis
Cellular and Molecular Life Sciences (2019)
-
Intestinal regulation of suppression of tumorigenicity 14 (ST14) and serine peptidase inhibitor, Kunitz type -1 (SPINT1) by transcription factor CDX2
Scientific Reports (2018)
-
The miR-487b-3p/GRM3/TGFβ signaling axis is an important regulator of colon cancer tumorigenesis
Oncogene (2017)
-
Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression
Oncogene (2017)