Abstract
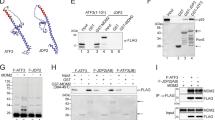
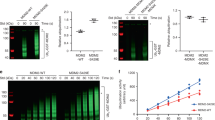
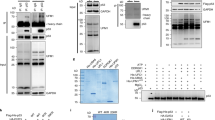
Key to p53 ability to mediate its multiple cellular functions lies in its stability. In the present study we have elucidated the mechanism by which Mdm2 regulates p53 degradation. Using in vitro and in vivo ubiquitination assays we demonstrate that Mdm2 association with p53 targets p53 ubiquitination. Exposure of cells to UV-irradiation inhibits this targeting. Mdm2 which is deficient in p53 binding failed to target p53 ubiquitination, suggesting that the association is essential for Mdm2 targeting ability. While mdm2-p53 complex is found in non-stressed cells, the amount of p53-bound mdm2 is decreased after UV-irradiation, further pointing to the relationship between mdm2 binding and p53 level. Similar to Swiss 3T3 cells, the dissociation of mdm2-p53 complex was also found in UV-treated Scid cells, lacking functional DNA-PK, suggesting that DNA-PK is not sufficient for dissociating mdm2 from p53. Together our studies point to the role of Mdm2, as one of p53-associated proteins, in targeting p53 ubiquitination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fuchs, S., Adler, V., Buschmann, T. et al. Mdm2 association with p53 targets its ubiquitination. Oncogene 17, 2543–2547 (1998). https://doi.org/10.1038/sj.onc.1202200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202200
Keywords
This article is cited by
-
1H, 15N and 13C backbone resonance assignments of the acidic domain of the human MDM2 protein
Biomolecular NMR Assignments (2023)
-
IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma
Oncogene (2022)
-
1H, 15N and 13C backbone resonance assignments of the acidic domain of the human MDMX protein
Biomolecular NMR Assignments (2022)
-
Scaling Synapses in the Presence of HIV
Neurochemical Research (2019)
-
Molecular mechanism of the TP53-MDM2-AR-AKT signalling network regulation by USP12
Oncogene (2018)