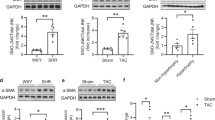
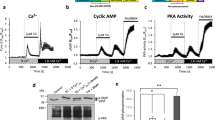
Abstract
We have previously demonstrated that arachidonic acid activates extracellular signal-regulated protein kinases (ERKs) group of mitogen-activated protein kinases (MAPKs) in vascular smooth muscle cells (VSMC). To understand the role of arachidonic acid in cellular signaling events, we have now studied its effect on jun N-terminal kinases (JNKs) group of MAPKs in VSMC. Arachidonic acid activated JNK1 in a time- and concentration-dependent manner with maximum effects at 10 min and 50 μM. Induced activation of JNK1 by arachidonic acid is specific as other fatty acids such as linoleic and stearic acids had no such effect. Indomethacin and nordihydroguaiaretic acid (NDGA), potent inhibitors of the cyclooxygenase (COX) and the lipoxygenase (LOX)/monooxygenase (MOX) pathways, respectively, had no effect on arachidonic acid activation of JNK1 suggesting that the observed phenomenon is independent of its metabolism through either pathway. However, 12-hydroperoxyeicosatetraenoic acid (12-HpETE), the LOX metabolite of arachidonic acid significantly induced JNK1 activity. Protein kinase C (PKC) depletion by prolonged treatment of VSMC with phorbol 12-myristate 13-acetate (PMA) resulted in partial decrease in the responsiveness of JNK1 to arachidonic acid suggesting a role for both PKC-dependent and -independent mechanisms in the activation of JNK1 by this important fatty acid. On the other hand, the responsiveness of JNK1 to 12-HpETE was completely abolished in PKC-depleted cells, suggesting a major role for PKC in 12-HpETE-induced JNK1 activation. IL-1β and TNF-α activated JNK1 in a time-dependent manner with maximum effect at 10 min. Desensitization of JNK1 by arachidonic acid significantly reduced its responsiveness to both the cytokines. In addition, 4-bromophenacyl bromide (4-BPB), a potent and selective inhibitor of phospholipase A2 (PLA2), significantly attenuated the cytokine-induced activation of JNK1. Together, these results show that (1) arachidonic acid and its LOX metabolite, 12-HpETE, activate JNK1 in VSMC, (2) PKC-dependent and -independent mechanisms play a role in the activation of JNK1 by arachidonic acid and 12-HpETE, and (3) arachidonic acid mediates, at least partially, the cytokine-induced activation of JNK1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Madamanchi, N., Bukoski, R., Runge, M. et al. Arachidonic acid activates Jun N-terminal kinase in vascular smooth muscle cells. Oncogene 16, 417–422 (1998). https://doi.org/10.1038/sj.onc.1201551
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1201551
Keywords
This article is cited by
-
Glycosylated human oxyhaemoglobin activates nuclear factor‐κB and activator protein‐1 in cultured human aortic smooth muscle
British Journal of Pharmacology (2003)
-
Mechanisms of nordihydroguaiaretic acid-induced growth inhibition and apoptosis in human cancer cells
British Journal of Cancer (2002)
-
Assessment of the arachidonic acid content in foods commonly consumed in the American diet
Lipids (1998)