Abstract
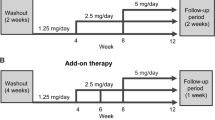
Patients with severe hypertension (>180/110 mm Hg) require large blood pressure (BP) reductions to reach recommended treatment goals (<140/90 mm Hg) and usually require combination therapy to do so. This 8-week, multicenter, randomized, double-blind, parallel-group study compared the tolerability and antihypertensive efficacy of the novel direct renin inhibitor aliskiren with the angiotensin converting enzyme inhibitor lisinopril in patients with severe hypertension (mean sitting diastolic blood pressure (msDBP)⩾105 mm Hg and <120 mm Hg). In all, 183 patients were randomized (2:1) to aliskiren 150 mg (n=125) or lisinopril 20 mg (n=58) with dose titration (to aliskiren 300 mg or lisinopril 40 mg) and subsequent addition of hydrochlorothiazide (HCTZ) if additional BP control was required. Aliskiren-based treatment (ALI) was similar to lisinopril-based treatment (LIS) with respect to the proportion of patients reporting an adverse event (AE; ALI 32.8%; LIS 29.3%) or discontinuing treatment due to AEs (ALI 3.2%; LIS 3.4%). The most frequently reported AEs in both groups were headache, nasopharyngitis and dizziness. At end point, ALI showed similar mean reductions from baseline to LIS in msDBP (ALI −18.5 mm Hg vs LIS −20.1 mm Hg; mean treatment difference 1.7 mm Hg (95% confidence interval (CI) −1.0, 4.4)) and mean sitting systolic blood pressure (ALI −20.0 mm Hg vs LIS −22.3 mm Hg; mean treatment difference 2.8 mm Hg (95% CI −1.7, 7.4)). Responder rates (msDBP<90 mm Hg and/or reduction from baseline⩾10 mm Hg) were 81.5% with ALI and 87.9% with LIS. Approximately half of patients required the addition of HCTZ to achieve BP control (ALI 53.6%; LIS 44.8%). In conclusion, ALI alone, or in combination with HCTZ, exhibits similar tolerability and antihypertensive efficacy to LIS alone, or in combination with HCTZ, in patients with severe hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252.
European Society of Hypertension–European Society of Cardiology. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens 2003; 21: 1011–1053.
Bloom BS . Continuation of initial antihypertensive medication after 1 year of therapy. Clin Ther 1998; 20: 671–681.
Burke TA, Sturkenboom MC, Lu SE, Wentworth CE, Lin Y, Rhoads GG . Discontinuation of antihypertensive drugs among newly diagnosed hypertensive patients in UK general practice. J Hypertens 2006; 24: 1193–1200.
Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366: 895–906.
Sica DA . Calcium channel blocker-related periperal edema: can it be resolved? J Clin Hypertens (Greenwich) 2003; 5: 291–294, 297.
Israili ZH, Hall WD . Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117: 234–242.
Dicpinigaitis PV . Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 169S–173S.
Azizi M, Menard J, Bissery A, Guyenne TT, Bura-Riviere A, Vaidyanathan S et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol 2004; 15: 3126–3133.
Skeggs Jr LT, Kahn JR, Lentz K, Shumway NP . The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med 1957; 106: 439–453.
Wood JM, Maibaum J, Rahuel J, Grutter MG, Cohen NC, Rasetti V et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun 2003; 308: 698–705.
Nussberger J, Wuerzner G, Jensen C, Brunner HR . Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension 2002; 39: E1–E8.
Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP . Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005; 111: 1012–1018.
Pool JL, Schmieder RE, Azizi M, Aldigier JC, Januszewicz A, Zidek W et al. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens 2007; 20: 11–20.
Villamil A, Chrysant S, Calhoun D, Schober B, Hsu H, Matrisciano-Dimichino L et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25: 217–226.
Sica D, Gradman AH, Lederballe O, Meyers M, Cai J, Keefe D . Aliskiren, a novel renin inhibitor, is well tolerated and has sustained BP-lowering effects alone or in combination with HCTZ during long-term (52 weeks) treatment of hypertension. Eur Heart J 2006; 27 (Suppl): 121.
Gossmann J, Thurmann P, Bachmann T, Weller S, Kachel HG, Schoeppe W et al. Mechanism of angiotensin converting enzyme inhibitor-related anemia in renal transplant recipients. Kidney Int 1996; 50: 973–978.
Rell K, Koziak K, Jarzyo I, Lao M, Gaciong Z . Correction of posttransplant erythrocytosis with enalapril. Transplantation 1994; 57: 1059–1063.
Midtvedt K, Hartmann A, Holdaas H, Fauchald P . Efficacy of nifedipine or lisinopril in the treatment of hypertension after renal transplantation: a double-blind randomised comparative trial. Clin Transplant 2001; 15: 426–431.
Radevski I, Skudicky D, Candy G, Sathekge S, Strugo V, Sareli P . Antihypertensive monotherapy with nisoldipine CC is superior to enalapril in black patients with severe hypertension. Am J Hypertens 1999; 12: 194–203.
Oparil S, Aurup P, Snavely D, Goldberg A . Efficacy and safety of losartan/hydrochlorothiazide in patients with severe hypertension. Am J Cardiol 2001; 87: 721–726.
Oparil S, Levine JH, Zuschke CA, Gradman AH, Ripley E, Jones DW et al. Effects of candesartan cilexetil in patients with severe systemic hypertension. Candesartan Cilexetil Study Investigators. Am J Cardiol 1999; 84: 289–293.
Acknowledgements
This study was supported by Novartis Pharma AG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registry: This trial is registered at ClinicalTrials.gov with trial identifier NCT00219050.
Rights and permissions
About this article
Cite this article
Strasser, R., Puig, J., Farsang, C. et al. A comparison of the tolerability of the direct renin inhibitor aliskiren and lisinopril in patients with severe hypertension. J Hum Hypertens 21, 780–787 (2007). https://doi.org/10.1038/sj.jhh.1002220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jhh.1002220
Keywords
This article is cited by
-
Aliskiren reduces home blood pressure and albuminuria in patients with hypertensive nephrosclerosis
Clinical and Experimental Nephrology (2013)
-
Factors correlated with plasma renin activity in general Japanese population
Clinical and Experimental Nephrology (2009)
-
Aliskiren, the first approved renin inhibitor: Clinical application and safety in the treatment of hypertension
Advances in Therapy (2009)
-
Direct renin inhibition: clinical pharmacology
Journal of Molecular Medicine (2008)
-
Renin inhibitors, clinical experience
Journal of Molecular Medicine (2008)