Abstract
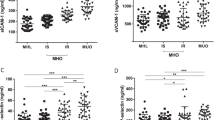
Hypertension and non insulin-dependent diabetes mellitus (NIDDM) are well-known risk factors for atherosclerotic disease. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) may exert a relevant role in the pathogenesis of atherosclerosis; their prognostic relevance has been recently demonstrated. The aim of the study was to investigate possible inter-relation between circulating adhesion molecule levels, carotid artery structure and endothelial function in 15 patients with NIDDM, as well as in 15 patients with both NIDDM and essential hypertension (NIDDM+EH) compared with 15 normal subjects (NS) and 15 euglycaemic patients with EH, matched for age, sex and body weight. All subjects were submitted to a biopsy of the gluteal subcutaneous fat. Small arteries were dissected and mounted on a micromyograph, and the media-to-lumen (M/L) ratio was then calculated. Carotid artery structure was investigated by Doppler ultrasound. Endothelial function was evaluated by investigation of the flow-mediated dilatation (FMD) of the brachial artery. ICAM-1 and VCAM-1 plasma levels were measured by ELISA. ICAM-1 and VCAM-1 plasma levels were significantly greater and FMD smaller in EH, NIDDM and NIDDM+EH than in NS, but no difference was observed among the three pathological groups. Carotid artery structural changes were more pronounced in NIDDM+EH. No significant difference was observed among NIDDM, EH and NS. The M/L ratio of subcutaneous small resistance arteries was significantly greater in NIDDM+EH than in NIDDM or EH. NS had a smaller M/L ratio than the other groups. Significant correlations were observed between ICAM-1 plasma levels and indices of carotid artery structure in diabetic patients. However, the relations were close only in NIDDM+EH. In conclusion, our data suggest that NIDDM+EH may present more pronounced vascular structural alterations than NIDDM, and that adhesion molecules plasma levels are closely inter-related with carotid artery structural alterations, at least in NIDDM+EH, but not with M/L ratio of small resistance arteries.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Adams DH, Shaw S . Leucocyte–endothelial interactions and regulation of leucocyte migration. Lancet 1994; 343: 831–836.
Martin SD, Springer TA . Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1. Cell 1987; 51: 813–819.
Dustin ML . Induction of IL-1 and IFN-γ: tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1). J Immunol 1986; 137: 245–254.
Bevilacqua MP . Endothelial–leucocyte adhesion molecules. Annu Rev Immunol 1993; 11: 767–804.
Cheng JJ, Wung BS, Chao YJ, Wang DL . Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension 1996; 28: 386–391.
DeSouza CA et al. Elevated levels of circulating cell adhesion molecules in uncomplicated essential hypertension. Am J Hypertens 1997; 10: 1335–1341.
Gasic S et al. Fosinopril decreases levels of soluble vascular cell adhesion molecule-1 in borderline hypertensive type II diabetic patients with microalbuminuria. Am J Hypertens 1999; 12: 217–222.
Steiner M, Reinhardt KM, Krammer B . Increased levels of soluble adhesion molecules in type 2 (non-insulin dependent) diabetes mellitus are independent of glycaemic control. Thromb Haemost 1994; 72: 979–984.
Wagner OF, Jilma B . Putative role of adhesion molecules in metabolic disorders. Horm Metab Res 1997; 29: 1–4.
Ferri C et al. Early activation of vascular endothelium in nonobese, nondiabetic essential hypertensive patients with multiple metabolic abnormalities. Diabetes 1998; 47: 660–667.
Rizzoni D et al. Endothelial dysfunction in small resistance arteries of patients with non-insulin dependent diabetes mellitus. J Hypertens 2001; 19: 913–919.
Winkelmann BR et al. Baseline levels of cell adhesion molecules P-selectin and ICAM-1 predicts 5-year sur-vival in patients with coronary artery disease. Abstracts from the 48th Annual Scientific Sessions American College of Cardiology. New Orleans. 7–10 March 1999. J Am Coll Cardiol 1999; 33 (Suppl A): 300A.
Wallen NH . Elevated serum intercellular adhesion molecule-1 and vascular adhesion molecule-1 among patients with stable angina pectoris who suffer cardiovascular death or non fatal myocardial infarction. Eur Heart J 1999; 20: 1039–1043.
Blankenberg S et al. for the StheroGene Investigators Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001; 104: 1336–1342.
Hwang S et al. Circulating adhesion molecules VCAM-1, ICAM-1 and E-selectin in carotid arterosclerosis and incident coronary artrery disease cases: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 1997; 96: 4219–4215.
Ridker P et al. Plasma concentration of soluble intercellular adhesion molecule-1 and risks of future myocardial infaction in apparently healthy men. Lancet 1998; 251: 88–92.
Malik I et al. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet 2001; 358: 971–975.
Ridker PM . Role of inflammatory biomarkers in prediction of coronary heart disease (Commentary). Lancet 2001; 358: 946–947.
Rizzoni D et al. Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 1996; 28: 785–790.
Taddei S, Virdis A, Mattei P, Salvetti A . Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension 1993; 21: 929–933.
Deng LY, Li JS, Schiffrin EL . Endothelium-dependent relaxation of small arteries from essential hypertensive patients: mechanisms and comparison with normotensive subjects and with responses of vessels from spontaneously hypertensive rats. Clin Sci 1995; 88: 611–622.
Goodfellow J et al. Endothelium and inelastic arteries: an early marker of vascular dysfunction in non-insulin dependent diabetes. BMJ 1996; 312: 744–745.
Parving HH et al. Macro-microangiopathy and endothelial dysfunction in NIDDM patients with and without diabetic nephropaty. Diabetologia 1996; 39: 1590–1597.
Rizzoni D et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non insulin dependent diabetes mellitus. Circulation 2001; 103: 1238–1244.
Celermajer DS et al. Endothelium-dependent dilatation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 1994; 24: 1468–1474.
Guidelines Subcommittee. 1999 World Health Organization—International Society of Hypertension Guidelines for the Management of Hypertension. J Hypertens 1999; 17: 151–183.
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classfication of Diabetes Mellitus. Diabetes Care 1997; 20: 1183–1201.
Porteri E et al. Effects of hypotensive and non-hypotensive doses of manidipine on structure, responses to endothelin-1 and ICAM-1 production in mesenteric small resistance arteries of spontaneously hypertensive rats. Blood Pressure 1999; 7: 324–330.
Pignoli P et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74: 1399–1406.
Rizzoni D et al. Relations between cardiac and vascular structure in patients with primary and secondary hypertension. J Am Coll Cardiol 1998; 32: 985–992.
Salonen R, Seppanen K, Rauramaa R, Salonen JT . Prevalence of carotid atherosclerosis and serum cholesterol levels in Eastern Finland. Atherosclerosis 1988; 8: 788–792.
Celermajer DS et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992; 340: 1111–1115.
Muiesan ML et al. Effect of treatment on flow dependent vasodilation of the brachial artery in essential hypertension. Hypertension 1999; 33 (Part II): 575–580.
Aalkjaer C et al. Evidence for increased media thickness, increased neural amine uptake, and depressed excitation-contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res 1987; 61: 181–186.
Mulvany MJ, Hansen PK, Aalkjaer C . Direct evidence that the greater contractility of resistance vessels in spontaneously hypertensive rats is associated with a narrowed lumen, a thickened media, and an increased number of smooth muscle cell layers. Circ Res 1978; 43: 854–864.
Vecchione C et al. Cardiovascular influences of α1B-adrenergic receptor in mice. Circulation 2002; 105: 1700–1707.
Mocco J et al. Elevation of soluble intercellular adhesion molecule-1 levels in symptomatic and asymptomatic carotid atherosclerosis. Neurosurgery 2001; 48: 718–721.
van der Meer I et al. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis. Arterioscler Thromb Vasc Biol 2002; 22: 838–842.
Blann AD, Farrel A, Picton A, McCollum CN . Relation between endothelial cell markers and arterial stenosis in peripheral and carotid artery disease. Thromb Res 2000; 97: 209–216.
Otsuki M et al. Circulating vascular cell adhesion molecule-1 (VCAM-1) in atherosclerotic NIDDM patients. Diabetes 1997; 46: 2096–2101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizzoni, D., Muiesan, M., Porteri, E. et al. Circulating adhesion molecules and carotid artery structural changes in patients with noninsulin-dependent diabetes mellitus. J Hum Hypertens 17, 463–470 (2003). https://doi.org/10.1038/sj.jhh.1001570
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jhh.1001570
Keywords
This article is cited by
-
Glucose and triglyceride excursions following a standardized meal in individuals with diabetes: ELSA-Brasil study
Cardiovascular Diabetology (2015)
-
Periodic vs constant high glucose in inducing pro-inflammatory cytokine expression in human coronary artery endothelial cells
Inflammation Research (2013)
-
Soluble integrin adhesion receptors and atherosclerosis: much heat and a little light?
Journal of Human Hypertension (2003)