Abstract
Objective:
To determine the relationship between body composition/fat distribution and parity after adjusting for potential confounders: age, smoking, and physical activity.
Design:
Cross-sectional.
Subjects:
A total of 170 Caucasian women between the ages of 18 and 76 years, who were non-smokers with no cardiovascular disease, diabetes, metabolic, or endocrine disorders.
Measurements:
Physical activity assessment (Baecke Physical Activity Questionnaire), anthropometric measures, and body composition (dual-energy X-ray absorptiometry, computed tomography).
Results:
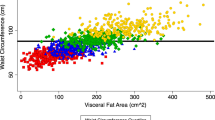
Although percent body fat was related to parity (r=0.26, P<0.01), after adjusting for age, physical activity index, and smoking, the parity–percent body fat relationship was no longer significant. Multiple regression analysis for modeling intra-abdominal adipose tissue demonstrated that parity and intra-abdominal adipose tissue were significantly related after adjusting for percent body fat, physical activity index, and smoking (partial r=0.18, P=0.02, unstandardized β=5.22±2.26, intercept=−37.32±24.63).
Conclusion:
Our data suggest that intra-abdominal adipose tissue increases with increasing parity, even after adjusting for potential confounders: age, percent body fat, physical activity, and smoking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
CDC. Morbidity and mortality weekly report; prevalence of overweight and obesity among adults with diagnosed diabetes-United States, 1988–1994 and 1999–2002. U.S. Government Printing Office (GPO): Washington, DC 2002; mmwrq@cdc.gov.
Herber D . The first name in women's health. http://www.drdonnica.com/guests/00001175.htm Accessed: March 2005.
Linne Y, Dye L, Barkeling B, Rossner S . Weight development over time in parous women – SPAWN study – 15 years follow-up. Int J Obes Relat Metab Disord 2003; 27: 1516–1522.
Davis SR . Identifying and promoting the specific nutrition and physical activity needs of women aged 40 and over. Summary Med J Aust 2000; 173 (Suppl 6 November): S89–S111.
Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, West DS, Sidney S . Long-term plasma lipid changes associated with a first birth. The CARDIA study. Am J Epidemiol 2004; 159: 1028–1039.
Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE . Longitudinal changes in adiposity associated with pregnancy. The CARDIA study. Coronary artery risk development in young adults. JAMA 1994; 271: 1747–1751.
Williamson DF, Madams J, Pamuk E, Flegal KM, Kendrick JS, Serdula MK . A prospective study if childbearing and 10-year weight gain in US white women 25–45 years of age. Int J Obes Relat Metab Disord 1994; 18: 561–569.
Soltani H, Frazer RB . A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutri 2000; 84: 95–101.
Sohlstrom A, Forsum E . Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr 1995; 61: 287–295.
Baecke JAH, Burema J, Fritters ER . A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982; 36: 936–942.
Jacobs DR, Ainsworth BE, Harman TJ, Leon AS . A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc 1993; 25: 81–91.
Mahoney M, Freedson P . Assessment of physical activity from caltrac and Baecke questionnaire techniques. Med Sci Sports Exerc 1990; 22: S80.
Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L . Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr 1988; 48: 1351–1361.
Borkan GA, Genzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE . Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 1982; 36: 172–177.
Toth MJ, Tchernof A, Sites CK, Poehlman ET . Menopause-related changes in body fat distribution. Ann NY Acad Sci 2000; 904: 502–506.
den Tonkelaar I, Seidell JC, van Noord PAH, Baanders-van Halewijn EA, Ouwehand IJ . Fat distribution in relation to age, degree of obesity, smoking habits, parity and estrogen use: a cross-sectional study in 11 825 Dutch women participating in the DOM-Project. Int J Obes Relat Metab Disord 1990; 14: 753–761.
Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE . Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr 2002; 75: 499–504.
Schoeller DA, Shay K, Kushner RF . How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr 1997; 66: 551–556.
Bamia C, Trichopoulou A, Lenas D, Trichopoulos D . Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Relat Metab Disord 2004; 28: 1091–1096.
Visser M, Launer LJ, Deurenberg P, Deeg DJ . Past and current smoking in relation to body fat distribution in older men and women. J Gerontol A Biol Sci Med Sci 1999; 54: M293–M298.
Barrett-Connor E, Khaw KT . Cigarette smoking and increased central adiposity. Ann Intern Med 1989; 111: 783–787.
Lawlor DA, Emberson JR, Ebrahim S, Whincup PH, Wannamethee SG, Walker M et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British women's heart and health study and the British regional heart study. Circulation 2003; 107: 1260–1264.
Ness RB, Schotland HM, Flegal KM, Shofer FS . Reproductive history and coronary heart disease risk in women. Epidemiol Rev 1994; 16: 298–314.
Dekker JM, Schouten EG . Number of pregnancies and risk of cardiovascular disease. N Engl J Med 1993; 329: 1893–1894.
Humphries KH, Westendorp ICD, Bots ML, Spinelli JJ, Carere RG, Hofman A et al. Parity and carotid artery atherosclerosis in elderly women. The Rotterdam Study. Stroke 2001; 32: 2259.
Russo J, Moral R, Balogh GA, Mailo D, Russo IH . The protective role of pregnancy in breast cancer. Breast Cancer Res 2005; 7: 131–142.
Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G . Breast cancer. Lancet 2005; 365: 1727–1741.
Epel E, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell K et al. Can stress shape your body? Consistently greater stress-induced cortisol secretion among women with abdominal fat. Psychosom Med 2000; 62: 623–632.
Larson DE, Hunter GR, Williams MJ, Kekes-Szabo T, Nyikos I, Goran MI . Dietary fat in relation to body fat and intraabdominal adipose tissue: a cross-sectional analysis. Am J Clin Nutr 1996; 64: 787–788.
Wannamethee SG, Shaper AG . Lifelong teetotallers, ex-drinkers and drinkers: mortality and the incidence of major coronary heart disease events in middle-aged British men. Int J Epidemiol 1997; 26: 523–531.
Dallongeville J, Marecaux N, Ducimetiere P, Ferrieres J, Arveile D, Bingham A et al. Influence of alcohol consumption and various beverages on waist girth and waist-to-hip ratio in a sample of French men and women. Int J Obes Relat Metab Disord 1998; 22: 1178–1183.
Austin MA . The Kaiser–Permanente women twins study data set. Genet Epidemiol 1993; 10: 519–522.
Laitinen J, Pietilainen K, Wadsworth M, Sovio U, Jarvelin M-R . Predictors of abdominal obesity among 31-y-old men and women born in Northern Finland in 1966. Eur J Clin Nutr 2004; 58: 180–190.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blaudeau, T., Hunter, G. & Sirikul, B. Intra-abdominal adipose tissue deposition and parity. Int J Obes 30, 1119–1124 (2006). https://doi.org/10.1038/sj.ijo.0803252
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803252
Keywords
This article is cited by
-
The association between parity and metabolic syndrome and its components in normal-weight postmenopausal women in China
BMC Endocrine Disorders (2021)
-
Ultrasound estimated subcutaneous and visceral adipose tissue thicknesses and risk of pre-eclampsia
Scientific Reports (2021)
-
Abdominal vs. overall obesity among women in a nutrition transition context: geographic and socio-economic patterns of abdominal-only obesity in Tunisia
Population Health Metrics (2015)
-
Parity and Risk of Coronary Heart Disease in Middle-aged and Older Chinese Women
Scientific Reports (2015)
-
Metabolic and genetic predictors of circulating adipocyte fatty acid-binding protein
International Journal of Obesity (2012)