Abstract
OBJECTIVE:
This study was conducted to elucidate whether antagonistic targeting of the histamine H3 receptor increases hypothalamic histamine levels, in parallel with decreases in food intake and body weight.
METHODS:
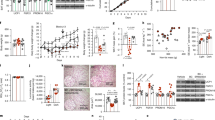
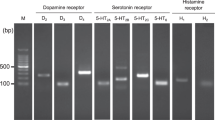
The competitive antagonist potency of a recently synthesized histamine H3 receptor antagonist, NNC 38-1049, was studied in intact HEK293 cells expressing human or rat histamine H3 receptor, in which NNC 38-1049 was allowed to antagonize the effect of the H3 receptor agonist R-α-methylhistamine on isoprenaline-induced accumulation of cAMP. The affinity of NNC 38-1049 for a number of variants of the histamine receptor was also determined. Following single dosing of normal rats with NNC 38-1049, hypothalamic histamine levels were assessed by means of microdialysis. Plasma and brain levels of NNC 38-1049 and acute effects on food intake and energy expenditure were followed after oral doses of 3–60 mg/kg. Potential side effects were examined with rat models of behaviour satiety sequence (BSS), pica behaviour and conditioned taste aversion (CTA). Intakes of food and water together with body weight were recorded for 15 days during daily dosing of dietary obese rats.
RESULTS:
NNC 38-1049 was found to be a highly specific and competitive antagonist towards both human and rat histamine H3 receptors, and measurable amounts of NNC 38-1049 were found in the plasma of rats following single oral doses of 3–60 mg/kg and in the brain after 15–60 mg/kg. Following single intraperitoneal injections of NNC 38-1049 (20 mg/kg), significant increases in extracellular histamine concentrations were observed. The same dose did not change BSS or pica behaviour acutely, nor did it induce CTA following repeated administration for 7 days. Reductions in food intake were seen very soon after administration, and occurred in a dose-dependent fashion. Energy expenditure was unchanged, but the respiratory quotient (RQ) tended to decrease at higher doses, indicating an increase in lipid oxidation. Twice daily administration of 20 mg/kg of NNC 38-1049 in old and dietary obese rats resulted in sustained reduction of food intake throughout a 2-week study, and was associated with a highly significant (P<0.01) decrease in body weight compared with controls (−18.4±3.4 vs +0.4±2.7 g). The same dose of NNC 38-1049 produced an acute decrease of water intake, but 24 h intakes were not significantly changed.
CONCLUSIONS:
The results of this study strongly support the idea that an increase in the hypothalamic concentration of histamine produces a specific reduction of food intake and that this effect can be translated into a decrease in body weight.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Sakata T, Ookuma K, Fujimoto K, Fukagawa K, Yoshimatsu H . Histaminergic control of energy balance in rats. Brain Res Bull 1991; 3–4: 371–375.
Fujise T, Yoshimatsu H, Kurokawa M, Oohara A, Kang M, Nakata M, Sakata T . Satiation and masticatory function modulated by brain histamine in rats. Proc Soc Exp Biol Med 1998; 2: 228–234.
Tsuda K, Yoshimatsu H, Niijima A, Chiba S, Okeda T, Sakata T . Hypothalamic histamine neurons activate lipolysis in rat adipose tissue. Exp Biol Med (Maywood) 2002; 3: 208–213.
Masaki T, Chiba S, Yoshimichi G, Yasuda T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H . Neuronal histamine regulates food intake, adiposity, and uncoupling protein expression in agouti yellow (A(y)/a) obese mice. Endocrinology 2003; 6: 2741–2748.
Mercer LP, Kelley DS, Humphries LL, Dunn JD . Manipulation of central nervous system histamine or histaminergic receptors (H1) affects food intake in rats. J Nutr 1994; 7: 1029–1036.
Mercer LP . Histamine and the neuroregulation of food intake. Nutrition 1997; 6: 581–582.
Vaziri P, Dang K, Anderson GH . Evidence for histamine involvement in the effect of histidine loads on food and water intake in rats. J Nutr 1997; 8: 1519–1526.
Morimoto T, Yamamoto Y, Yamatodani A . Leptin facilitates histamine release from the hypothalamus in rats. Brain Res 2000; 2: 367–369.
Toftegaard CL, Knigge U, Kjaer A, Warberg J . The role of hypothalamic histamine in leptin-induced suppression of short-term food intake in fasted rats. Regul Pept 2003; 1–3: 83–90.
Rushing PA, Hagan MM, Seeley RJ, Lutz TA, D'Alessio DA, Air EL, Woods SC . Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology 2001; 11: 5035.
Mollet A, Lutz TA, Meier S, Riediger T, Rushing PA, Scharrer E . Histamine H1 receptors mediate the anorectic action of the pancreatic hormone amylin. Am J Physiol Regul Integr Comp Physiol 2001; 5: R1442–R1448.
Kent P, Plamondon H, Merali Z . Pharmaco-ontogeny of bombesin's suppression of food intake and its attenuation by histamine H3 receptor agonists. Brain Res Dev Brain Res 1997; 1: 87–95.
Lecklin A, Etu-Seppala P, Stark H, Tuomisto L . Effects of intracerebroventricularly infused histamine and selective H1, H2 and H3 agonists on food and water intake and urine flow in Wistar rats. Brain Res 1998; 1–2: 279–288.
Haq AU, Bundrant HM, Mercer LP . Food intake is inversely correlated with central nervous system histamine receptor (H1) concentrations in male Sprague–Dawley rats fed normal, low protein, low energy or poor quality protein diets. J Nutr 1996; 12: 3083–3089.
Arrang JM, Garbarg M, Schwartz JC . Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983; 5911: 832–837.
Arrang JM, Garbarg M, Schwartz JC . Autoregulation of histamine release in brain by presynaptic H3-receptors. Neuroscience 1985; 2: 553–562.
Zaragoza F, Stephensen H, Knudsen SM, Pridal L, Wulff BS, Rimvall K . 1-Alkyl-4-acylpiperazines as a new class of imidazole-free histamine H(3) receptor antagonists. J Med Chem 2004; 11: 2833–2838.
Wulff BS, Hastrup S, Rimvall K . Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur J Pharmacol 2002; 1: 33–41.
Malmlöf K, Johansen T . Growth hormone-mediated breakdown of body fat: insulin and leptin responses to GH are modulated by diet composition and caloric intake in old rats. Hormone Metab Res (Hormon Stoffwechselforsch Hormones Metab) 2003; 4: 236–242.
Halford JC, Blundell JE . Metergoline antagonizes fluoxetine-induced suppression of food intake but not changes in the behavioural satiety sequence. Pharmacol Biochem Behav 1996; 4: 745–751.
Ookuma K, Yoshimatsu H, Sakata T, Fujimoto K, Fukagawa F . Hypothalamic sites of neuronal histamine action on food intake by rats. Brain Res 1989; 2: 268–275.
Palacios JM, Wamsley JK, Kuhar MJ . The distribution of histamine H1-receptors in the rat brain: an autoradiographic study. Neuroscience 1981; 1: 15–37.
Itoh Y, Oishi R, Nishibori M, Saeki K . Characterization of histamine release from the rat hypothalamus as measured by in vivo microdialysis. J Neurochem 1991; 3: 769–774.
Jansen FP, Mochizuki T, Yamamoto Y, Timmerman H, Yamatodani A . In vivo modulation of rat hypothalamic histamine release by the histamine H3 receptor ligands, immepip and clobenpropit. Effects of intrahypothalamic and peripheral application. Eur J Pharmacol 1998; 2–3: 149–155.
Westerink BH, Cremers TI, De Vries JB, Liefers H, Tran N, De Boer P . Evidence for activation of histamine H3 autoreceptors during handling stress in the prefrontal cortex of the rat. Synapse 2002; 4: 238–243.
Doi T, Sakata T, Yoshimatsu H, Machidori H, Kurokawa M, Jayasekara LA, Niki N . Hypothalamic neuronal histamine regulates feeding circadian rhythm in rats. Brain Res 1994; 2: 311–318.
Itoh E, Fujimiya M, Inui A . Thioperamide, a histamine H3 receptor antagonist, powerfully suppresses peptide YY-induced food intake in rats. Biol Psychiatry 1999; 4: 475–481.
Attoub S, Moizo L, Sobhani I, Laigneau JP, Lewin MJ, Bado A . The H3 receptor is involved in cholecystokinin inhibition of food intake in rats. Life Sci 2001; 4: 469–478.
Hancock AA, Bennani YL, Bush EN, Esbenshade TA, Faghih R, Fox GB, Jacobson P, Knourek-Segel V, Krueger KM, Nuss ME, Pan JB, Shapiro R, Witte DG, Yao BB . Antiobesity effects of A-331440, a novel non-imidazole histamine H3 receptor antagonist. Eur J Pharmacol 2004; 1–3: 183–197.
De Beun R, Lohmann A, Schneider R, De Vry J . Ethanol intake-reducing effects of ipsapirone in rats are not due to simple stimulus substitution. Pharmacol Biochem Behav 1996; 4: 891–898.
Takahashi K, Suwa H, Ishikawa T, Kotani H . Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J Clin Invest 2002; 12: 1791–1799.
Sindelar DK, Shepperd ML, Pickard RT, Alexander-Chacko J, Dill MJ, Cramer JW, Smith DP, Gadski R . Central H3R activation by thioperamide does not affect energy balance. Pharmacol Biochem Behav 2004; 2: 275–283.
Tsuda K, Yoshimatsu H, Niijima A, Chiba S, Okeda T, Sakata T . Hypothalamic histamine neurons activate lipolysis in rat adipose tissue. Exp Biol Med (Maywood) 2002; 3: 208–213.
Kjaer A, Larsen PJ, Knigge U, Warberg J . Histaminergic activation of the hypothalamic–pituitary–adrenal axis. Endocrinology 1994; 3: 1171–1177.
Leurs R, Bakker RA, Timmerman H, de Esch IJ . The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov 2005; 2: 107–120.
Acknowledgements
We acknowledge the late Dr René de Beun for initiating the BSS and pica studies. The excellent technical assistance provided by Mr Frank Strauss and Mrs Hanne Jepsen is particularly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Parts of this work were presented in abstract form at the 12th European Congress of Obesity in Helsinki 2003, Finland, and at the European Histamine Research Society 2003 meeting in Noordwijkerhout, the Netherlands.
Rights and permissions
About this article
Cite this article
Malmlöf, K., Zaragoza, F., Golozoubova, V. et al. Influence of a selective histamine H3 receptor antagonist on hypothalamic neural activity, food intake and body weight. Int J Obes 29, 1402–1412 (2005). https://doi.org/10.1038/sj.ijo.0803036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0803036