Abstract
OBJECTIVE:
Isomerized hop extract (IHE), which consists mainly of isohumulones and is required in the beer brewing process, was investigated for its effects on diet-induced obesity in two strains of mice.
DESIGN:
C57BL/6N and KK-Ay mice were fed a standard or high-fat diet containing IHE and their body and tissue weights were measured at various time points. Oral glucose tolerance tests (OGTT) and insulin tolerance tests (ITT) were carried out in high-fat diet-fed C57BL/6N mice. The effects of IHE on intestinal lipid absorption were examined in Wistar rats using a plasma triacylglycerol assay after oral administration of a lipid emulsion. Fecal lipid levels were also measured in these animals after they were fed a high-fat diet containing IHE for 15 days. The effects of IHE on pancreatic lipase activity and the expression of genes involved in hepatic lipid metabolism were also examined using an in vitro assay and quantitative RT-PCR, respectively.
RESULTS:
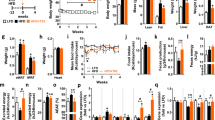
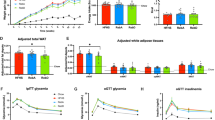
Supplementation of high-fat-containing chow with IHE reduced body weight gain and improved glucose tolerance in our experimental mice. A reduction in body weight gain was also observed in C57BL/6N mice fed a standard diet containing IHE. Wistar rats fed a high-fat diet containing IHE showed reduced plasma triacylglycerol levels and an increase in their fecal lipid excretion. Similarly, their pancreatic lipase activity was inhibited and their elevation in plasma triacylglycerol levels seen after the oral administration of lipid emulsion was significantly suppressed. IHE-fed mice showed an increased expression in their lipid oxidation genes and a decreased expression in genes involved in triacylglycerol biosynthesis.
CONCLUSION:
The inhibition of intestinal dietary fat absorption may be the mechanism by which IHE induces its weight-lowering effects in high-fat diet-fed mice. The modulatory effect of IHE on lipid metabolism may also, at least partly, be responsible for its beneficial effects on body weight gain. These results suggest that IHE may be helpful in humans in preventing diet-induced obesity and perhaps even metabolic syndrome, the latter of which is known to be associated with obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Spiegelman BM, Flier JS . Obesity and the regulation of energy balance. Cell 2001; 104: 531–543.
Kahn BB, Flier JS . Obesity and insulin resistance. J Clin Invest 2000; 106: 473–481.
Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T . Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Biochem 2001; 65: 2735–2740.
Han LK, Takaku T, Li J, Kimura Y, Okuda H . Anti-obesity action of oolong tea. Int J Obes Relat Metab Disord 1999; 23: 98–105.
Birketvedt GS, Travis A, Langbakk B, Florholmen JR . Dietary supplementation with bean extract improves lipid profile in overweight and obese subjects. Nutrition 2002; 18: 729–733.
Shimamura M, Hazato T, Ashino H, Yamamoto Y, Iwasaki E, Tobe H, Yamamoto K, Yamamoto S . Inhibition of angiogenesis by humulone, a bitter acid from beer hop. Biochem Biophys Res Commun 2001; 289: 220–224.
Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW . Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 2001; 50: 411–417.
Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, Berge RK, Staels B . Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem 2000; 275: 16638–16642.
Yajima H, Ikeshima E, Shiraki M, Kanaya T, Fujiwara D, Odai H, Tsuboyama-Kasaoka N, Ezaki O, Oikawa S, Kondo K . Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor α and γ and reduce insulin resistance. J Biol Chem 2004; 279: 33456–33462.
Reeves PG, Nielsen FH, Fahey Jr GC . AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939–1951.
Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O . High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism 1996; 45: 1539–1546.
Carr TP, Andresen CJ, Rudel LL . Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem 1993; 26: 39–42.
Folch J, Lees M, Sloane-Stanley GH . A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 1957; 226: 497–509.
Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y . Saponins from Platycodi radix ameliorate high fat diet-induced obesity in mice. J Nutr 2002; 132: 2241–2245.
Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA . Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1997; 272: 3406–3410.
Cohen B, Barkan D, Levy Y, Goldberg I, Fridman E, Kopolovic J, Rubinstein M . Leptin induces angiopoietin-2 expression in adipose tissues. J Biol Chem 2001; 276: 7697–7700.
Laborda J . 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res 1991; 19: 3998.
Han LK, Kimura Y, Kawashima M, Takaku T, Taniyama T, Hayashi T, Zheng YN, Okuda H . Anti-obesity effects in rodents of dietary teasaponin, a lipase inhibitor. Int J Obes Relat Metab Disord 2001; 25: 1459–1464.
Schoonjans K, Staels B, Auwerx J . Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 1996; 37: 907–925.
Waterman IJ, Zammit VA . Differential effects of fenofibrate or simvastatin treatment of rats on hepatic microsomal overt and latent diacylglycerol acyltransferase activities. Diabetes 2002; 51: 1708–1713.
Meegalla RL, Billheimer JT, Cheng D . Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun 2002; 298: 317–323.
Nicholls DG, Locke RM . Thermogenic mechanisms in brown fat. Physiol Rev 1984; 64: 1–64.
Acknowledgements
We thank K Sato and K Akatsuka for their hands-on assistance with the above experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yajima, H., Noguchi, T., Ikeshima, E. et al. Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int J Obes 29, 991–997 (2005). https://doi.org/10.1038/sj.ijo.0802965
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802965
Keywords
This article is cited by
-
Matured hop bitter acids improve spatial working and object recognition memory via nicotinic acetylcholine receptors
Psychopharmacology (2019)
-
The relationship between the effect of matured hop extract and physical activity on reducing body fat: re-analysis of data from a randomized, double-blind, placebo-controlled parallel group study
Nutrition Journal (2018)
-
Matured Hop-Derived Bitter Components in Beer Improve Hippocampus-Dependent Memory Through Activation of the Vagus Nerve
Scientific Reports (2018)
-
Iso-α-acids, bitter components of beer, prevent obesity-induced cognitive decline
Scientific Reports (2018)
-
Iso-alpha acids from hops (Humulus lupulus) inhibit hepatic steatosis, inflammation, and fibrosis
Laboratory Investigation (2018)