Abstract
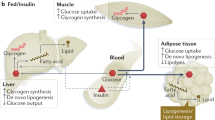
This review postulates and presents recent evidence that insulin resistance is initiated in the adipose tissue and also suggests that the adipose tissue may play a pivotal role in the induction of insulin resistance in the muscles and the liver. Marked impairments in insulin's intracellular signaling cascade are present in fat cells from type 2 diabetic patients, including reduced IRS-1 gene and protein expression, impaired insulin-stimulated PI3-kinase and PKB/Akt activities. In contrast, upstream insulin signaling in skeletal muscle from diabetic subjects only shows modest impairments and PKB/Akt activation in vivo by insulin appears normal. However, insulin-stimulated glucose transport and glycogen synthesis are markedly reduced.
Similar marked impairments in insulin signaling, including reduced IRS-1 expression, impaired insulin-stimulated PI3-kinase and PKB/Akt activities are also seen in some (∼30%) normoglycemic individuals with genetic predisposition for type 2 diabetes. In addition, GLUT4 expression is markedly reduced in these cells, similar to what is seen in diabetic cells. The individuals with reduced cellular expression of IRS-1 and GLUT4 are also markedly insulin resistant and exhibit several characteristics of the Insulin Resistance Syndrome.
Thus, a ‘diabetic’ pattern is seen in the fat cells also in normoglycemic subjects and this is associated with a marked insulin resistance in vivo. It is proposed that insulin resistance and/or its effectors is initiated in fat cells and that this may secondarily encompass other target tissues for insulin, including the impaired glucose transport in the muscles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
DeFronzo RA, Bonadonna RC, Ferrannini E . Pathogenesis of NIDDM. A balanced overview Diabetes Care 1992 15: 318–368.
Araki E, Lipes MA, Patti ME, Bruning JC, Haag B III, Johnson RS, Kahn CR . Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene Nature 1994 372: 186–190.
Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF . Disruption of IRS-2 causes type 2 diabetes in mice Nature 1998 391: 900–904.
Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB . Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance Nature Med 2000 6: 924–928.
Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB . Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver Nature 2001 409: 729–733.
Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI . Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle J Clin Invest 2000 105: 1791–1797.
Zierath JR, Krook A, Wallberg-Henriksson H . Insulin action and insulin resistance in human skeletal muscle. [In Process Citation] Diabetologia 2000 43: 821–835.
Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB . Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. [See comments.] J Clin Invest 1999 104: 733–741.
Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H . Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects Diabetes 1998 47: 1281–1286.
Wu X, Sallinen K, Anttila L, Makinen M, Luo C, Pollanen P, Erkkola R . Expression of insulin-receptor substrate-1 and -2 in ovaries from women with insulin resistance and from controls Fertil Steril 2000 74: 564–572.
Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF . Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways J Clin Invest 2001 107: 181–189.
Zierath JR, He L, Guma A, Odegoard Wahlstrom E, Klip A, Wallberg-Henriksson H . Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM Diabetologia 1996 39: 1180–1189.
Zierath JR, Galuska D, Nolte LA, Thorne A, Kristensen JS, Wallberg-Henriksson H . Effects of glycaemia on glucose transport in isolated skeletal muscle from patients with NIDDM: in vitro reversal of muscular insulin resistance Diabetologia 1994 37: 270–277.
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM . Tumor necrosis factor alpha inhibits signaling from the insulin receptor Proc Natl Acad Sci USA 1994 91: 4854–4858.
Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI . Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity J Clin Invest 1999 103: 253–259.
Pedersen O, Bak JF, Andersen PH, Lund S, Moller DE, Flier JS, Kahn BB . Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM Diabetes 1990 39: 865–870.
Rondinone CM, Wang LM, Lonnroth P, Wesslau C, Pierce JH, Smith U . Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus Proc Natl Acad Sci USA 1997 94: 4171–4175.
Carvalho E, Eliasson B, Wesslau C, Smith U . Impaired phosphorylation and insulin-stimulated translocation to the plasma membrane of protein kinase B/Akt in adipocytes from type II diabetic subjects. [In Process Citation.] Diabetologia 2000 43: 1107–1115.
Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A . Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM Diabetes 1992 41: 465–475.
Vaag A, Henriksen JE, Beck-Nielsen H . Decreased insulin activation of glycogen synthase in skeletal muscles in young nonobese Caucasian first-degree relatives of patients with non-insulin-dependent diabetes mellitus J Clin Invest 1992 89: 782–788.
Handberg A, Vaag A, Vinten J, Beck-Nielsen H . Decreased tyrosine kinase activity in partially purified insulin receptors from muscle of young, non-obese first degree relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus Diabetologia 1993 36: 668–674.
Pratipanawatr W, Pratipanawatr T, Cusi K, Berria R, Adams JM, Jenkinson CP, Maezono K, DeFronzo RA, Mandarino LJ . Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation Diabetes 2001 50: 2572–2578.
Storgaard H, Song XM, Jensen CB, Madsbad S, Bjornholm M, Vaag A, Zierath JR . Insulin signal transduction in skeletal muscle from glucose-intolerant relatives with type 2 diabetes Diabetes 2001 50: 2770–2778.
Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ . Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle J Clin Invest 2000 105: 311–320.
Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL . Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects J Clin Invest 1995 95: 2195–2204.
Krutzfeldt J, Kausch C, Volk A, Klein HH, Rett K, Haring HU, Stumvoll M . Insulin signaling and action in cultured skeletal muscle cells from lean healthy humans with high and low insulin sensitivity Diabetes 2000 49: 992–998.
Mott DM, Pratley RE, Bogardus C . Postabsorptive respiratory quotient and insulin-stimulated glucose storage rate in nondiabetic Pima Indians are related to glycogen synthase fractional activity in cultured myoblasts J Clin Invest 1998 101: 2251–2256.
Carvalho E, Jansson PA, Axelsen M, Eriksson JW, Huang X, Groop L, Rondinone C, Sjostrom L, Smith U . Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM FASEB J 1999 13: 2173–2178.
Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U . Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport FASEB J 2001 15: 1101–1103.
Morris RD, Rimm DL, Hartz AJ, Kalkhoff RK, Rimm AA . Obesity and heredity in the etiology of non-insulin-dependent diabetes mellitus in 32,662 adult white women Am J Epidemiol 1989 130: 112–121.
Lapidus L, Bengtsson C, Lissner L, Smith U . Family history of diabetes in relation to different types of obesity and change of obesity during 12-yr period. Results from prospective population study of women in Goteborg, Sweden Diabetes Care 1992 15: 1455–1458.
Grill V, Persson G, Carlsson S, Norman A, Alvarsson M, Ostensson CG, Svanstrom L, Efendic S . Family history of diabetes in middle-aged Swedish men is a gender unrelated factor which associates with insulinopenia in newly diagnosed diabetic subjects Diabetologia 1999 42: 15–23.
Reaven GM . Banting lecture 1988. Role of insulin resistance in human disease Diabetes 1988 37: 1595–1607.
Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI . Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. [See comments.] New Engl J Med 1999 341: 240–246.
Ricort JM, Tanti JF, Van Obberghen E, Le Marchand-Brustel Y . Alterations in insulin signalling pathway induced by prolonged insulin treatment of 3T3-L1 adipocytes Diabetologia 1995 38: 1148–1156.
Chiang SH, Baumann CA, Kanzaki M, Thurmond DC, Watson RT, Neudauer CL, Macara IG, Pessin JE, Saltiel AR . Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10 Nature 2001 410: 944–948.
Stephens JM, Lee J, Pilch PF . Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction J Biol Chem 1997 272: 971–976.
Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, Moss LG, Greenberg AS . Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes J Biol Chem 1998 273: 24665–24669.
Gasic S, Tian B, Green A . Tumor necrosis factor alpha stimulates lipolysis in adipocytes by decreasing Gi protein concentrations J Biol Chem 1999 274: 6770–6775.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA . The hormone resistin links obesity to diabetes Nature 2001 409: 307–312.
Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV Jr, Shelton DL, Hebert CC . FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family EMBO J 2000 19: 4046–4055.
Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, O'Rahilly S . Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans Diabetes 2001 50: 2199–2202.
Nagaev I, Smith U . Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle Biochem Biophys Res Commun 2001 285: 561–564.
Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL . Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy Nature 1999 401: 73–76.
Unger RH, Orci L . Diseases of liporegulation: new perspective on obesity and related disorders FASEB J 2001 15: 312–321.
Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML . Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice J Clin Invest 2000 105: 271–278.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y . PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein Diabetes 2001 50: 2094–2099.
Halleux CM, Takahashi M, Delporte ML, Detry R, Funahashi T, Matsuzawa Y, Brichard SM . Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue Biochem Biophys Res Commun 2001 288: 1102–1107.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T . The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity Nature Med 2001 7: 941–946.
Olefsky JM . Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists J Clin Invest 2000 106: 467–472.
Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B . Thiazolidinediones (PPARgamma agonists) but not PPARalpha agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes FASEB J 2001 15: 215–220.
Patti ME, Sun XJ, Bruening JC, Araki E, Lipes MA, White MF, Kahn CR . 4PS/insulin receptor substrate (IRS)-2 is the alternative substrate of the insulin receptor in IRS-1-deficient mice J Biol Chem 1995 270: 24670–24673.
Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL . Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice Mol Cell 2000 6: 77–86.
Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, Satoh S, Sekihara H, Sciacchitano S, Lesniak M, Aizawa S, Nagai R, Kimura S, Akanuma Y, Taylor SI, Kadowaki T . Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia Diabetes 2000 49: 1880–1889.
Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF . Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling Nature Genet 1999 23: 32–40.
Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, Terauchi Y, Kamon J, Kaburagi Y, Matsui J, Akanuma Y, Nagai R, Kimura S, Tobe K, Kadowaki T . Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation Mol Cell Biol 2001 21: 2521–2532.
Rosen ED, Spiegelman BM . PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth J Biol Chem 2001 276: 37731–37734.
Peraldi P, Xu M, Spiegelman BM . Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling J Clin Invest 1997 100: 1863–1869.
Rahn T, Ridderstrale M, Tornqvist H, Manganiello V, Fredrikson G, Belfrage P, Degerman E . Essential role of phosphatidylinositol 3-kinase in insulin-induced activation and phosphorylation of the cGMP-inhibited cAMP phosphodiesterase in rat adipocytes. Studies using the selective inhibitor wortmannin FEBS Lett 1994 350: 314–318.
Acknowledgements
The studies performed in the author's laboratory were supported by grants from the Swedish Medical Research Council (project B-3506), the Swedish Diabetes Association, the European Community (QLG1-CT-1999-00674), Gullan and Sven-Erik Karlsson, the Sonya Hedenbratt Memorial Fund and the IngaBritt and Arne Lundberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, U. Impaired (‘diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance—is insulin resistance initiated in the adipose tissue?. Int J Obes 26, 897–904 (2002). https://doi.org/10.1038/sj.ijo.0802028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802028
Keywords
This article is cited by
-
Liver, visceral and subcutaneous fat in men and women of South Asian and white European descent: a systematic review and meta-analysis of new and published data
Diabetologia (2023)
-
H19 inhibition increases HDAC6 and regulates IRS1 levels and insulin signaling in the skeletal muscle during diabetes
Molecular Medicine (2022)
-
NLRP3 deficiency did not attenuate NASH development under high fat calorie diet plus high fructose and glucose in drinking water
Laboratory Investigation (2021)
-
Manipulation of intestinal microbiome as potential treatment for insulin resistance and type 2 diabetes
European Journal of Nutrition (2021)
-
Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism
Molecular Biology Reports (2020)