Abstract
OBJECTIVES: To compare the effects of 18 months of continuous vs intermittent exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary, moderately obese females.
DESIGN: Randomized, prospective, long-term cohort study. Subjects performed continuous exercise at 60–75% of maximum aerobic capacity, 3 days per week, 30 min per session, or exercised intermittently using brisk walking for two, 15 min sessions, 5 days per week.
MEASURES: Aerobic capacity, body weight, body composition, and metabolic fitness (blood pressure, lipids, glucose and insulin).
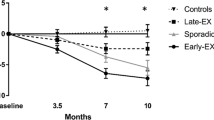
RESULTS: Significant improvements for aerobic capacity of 8% and 6% were shown for the continuous and intermittent exercise groups, respectively. Weight loss for the continuous exercise group was significant at 2.1% from baseline weight and the intermittent group was essentially unchanged. The continuous group showed a significant decrease in percentage of body fat and fat weight while the intermittent group did not. HDL cholesterol and insulin were significantly improved for both groups.
CONCLUSIONS: In previously sedentary, moderately obese females, continuous or intermittent exercise performed long-term may be effective for preventing weight gain and for improving some measures of metabolic fitness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL . Increasing prevalence of overweight among US adults JAMA 1994 272 (3): 205–211.
US Department of Health and Human Services . P. Healthy People 2000: National health promotion and disease prevention objectives. DHHS Publication No. (PHS) 91-50212 Washington DC: US Government Printing Office, Public Health Service 1990.
James WP . What are the health risks? The medical consequences of obesity and its health risks Exp Clin Endocrinol Diabetes 1998 106 (S2): 1–6.
Stunkard AJ, LaFleur WR, Wadden TA . Stimatization of obesity in medieval times: Asia and Europe Int J Obes 1998 22: 1141–1144.
Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH . Social and economic consequences of overweight in adolescence and young adulthood N Engl J Med 1993 329 (14): 1008–1012.
Dattilo AM, Kris-Etherton PM . Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis Am J Clin Nutr 1992 56: 320–328.
Garrison RJ, Castelli WP . Weight and thirty-year mortality of men in the framingham study Annals of Internal Medicine 1985 103: 1006–1009.
Stunkard A, McLaren-Hume M . The results of treatment for obesity Arch Intern Med 1959 103: 79–85.
Kayman S, Bruvold W, Stern JS . Maintenance and relapse after weight loss in women: behavioral aspects Am J Clin Nutr 1990 52: 800–807.
Ballor DL, Keesey RE . A meta-analysis of the factors affecting exercise-induced changes in body mass, fat mass, and fat-free mass in males and females Int J Obes 1991 15: 717–726.
Bouchard C, Depres JP, Tremblay A . Exercise and obesity Obes Res 1993 1: 133–147.
Jakicic JM, Polley BA, Wing RR . Accuracy of self-reported exercise and the relationship with weight loss in overweight women Med Sci Sports Exerc 1998 30: 634–638.
American College of Sports Medicine . Guidelines for Exercise Testing and prescription. 3 ed Philadelphia: Lea & Febiger 1988.
Pate RR, Pratt M, Blair SN, et al.Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine JAMA 1995 273 (5): 402–407.
Gortmaker SL, Dietz WH, Cheung LW . Inactivity, diet, and the fattening of America J Am Diet Assoc 1990 90: 1247–1255.
Sallis JF, Haskell WL, Wood PD, et al.Physical activity assessment methodology in the five-city project Am J Epidemiol 1985 121 (1): 91–106.
American Heart Association . Exercise testing and training of apparently healthy individuals: a handbook for physicians. The committee on exercise. Dallas, TX: American Heart Association, #70-008-A, 1972.
Snyder KA, Donnelly JE, Jacobsen DJ, Hertner G, Jakicic JM . The effects of long-term, moderate intensity, intermittent exercise on aerobic capacity, body composition, blood lipids, insulin, and glucose in overweight females Int J Obes 1997 21: 1180–1189.
Ballor DL, McCarthy JP, Wilterdink EJ . Exercise intensity does not effect the composition of diet- and exercise-induced body mass loss Am J Clin Nutr 1990 51: 142–146.
Van Dale D, Saris WHM, Schoffelen PFM, Ten Hoor F . Does exercise give an additional effect in weight reduction regimens Int J Obes 1987 11: 367–375.
Donnelly JE, Jacobsen DJ, Jakicic JM et al.Estimation of peak oxygen consumption from a sub-maximal half mile walk in obese females Int J Obes 1993 16: 585–589.
Joint National Committee . The fifth report of the Joint National Committee on detection, evaluation, and treatment of high blood pressure (JNC-V) Arch Intern Med 1993 153: 154–183.
Bonge D, Donnelly JE . Trials to criteria for hydrostatic weighing at residual volume Res Q Exerc Sport 1989 60: 176–179.
Wilmore JH, Vodak PA, Parr RB, Girandola RN, Billing JE . Further simplification of a method for determination of residual lung volume Med Sci Sports Exerc 1980 12 (3): 216–218.
Goldman RF, Buskirk ER . Body volume measurement by under-water weighing: description of a method. In: Brozek J, Henschel A, eds. Techniques for measuring body composition. Bethesda: National Academy of Sciences and National Research Council, 1993.
Brozek J, Grande F, Anderson JT, Keys A . Densitometric analysis of body composition: revision of some quantitative assumptions Ann NY Acad Sci 1963 110: 113–160.
Lohman TG, Roche AF, Martorell R . Anthropometric standardization reference manual. Champaign, III: Human Kinetics Books, 1988.
Andersen RE, Wadden TA, Bartlett SJ, Zemel D, Verde TL, Franckowiak SC . Effects of lifestyle activity vs structured aerobic exercise in obese women JAMA 1999 281: 335–340.
Burnstein M, Scholnick HR, Morfin R . Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions J Lip Res 1970 11: 583–595.
Morgan CR, Larner J . Immunoassay of insulin: two antibody system: plasma insulin levels of normal, subdiabetic rats Diabetes 1963 12: 115–126.
Lee RD, Neiman DC, Rainwater M . Comparison of eight microcomputer dietary analysis programs with the USDA nutrient data base for standard reference JADA 1995 95: 858–867.
Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW . Physical fitness and all-cause mortality JAMA 1989 262 (17): 2395–2401.
Wood PD, Haskell WL, Blair SN et al.Increased exercise level and plasma lipoprotein concentrations: A one-year, randomized, controlled study in sedentary, middle-aged men Metab 1983 32 (1): 31–39.
Wood PD, Terry RB, Haskell WL . Metabolism of substrates: diet, lipoprotein metabolism, and exercise Federation Proceedings 1985 44: 358–363.
Milesis CA, Pollock ML, Bah MD, Ayres J, Ward A, Linnerud AC . Effects of different duration of training on cardiorespiratory function, body composition and serum lipids Res Q Exerc Sport 1976 47: 716–725.
Gettman L, Pollock ML, Durstine JL, Ward A, Linnerud AC . Physiological responses of men to 1, 3, and 5 day per week training program Res Q Exerc Sport 1976 47: 638–646.
Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM . Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females Am J Clin Nutr 1991 54: 56–61.
Martin LJ, Su WF, Jones PH, Lockwood GA, Tritchler DL, Boyd NF . Comparison of energy intakes determined by food records and doubly labeled water in women participating in a dietary-intervention trial Am J Clin Nutr 1996 63 (4): 483–490.
Epstein LH, Wing RR . Exercise and weight Addictive Behaviors 1980 5: 371–388.
National Institutes of Health . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report Obes Res 1998 6 (2): 1S–210S.
Pronk NP, Wing RR . Physical activity and long-term maintenance of weight loss Obes Res 1994 2 (4): 587–599.
Blair SN, Kampert JB, Kohl HW, III et al.Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women JAMA 1996 276: 205–210.
Leon AS, Conrad J, Hunninghake DB, Serfass R . Effects of a vigorous walking program on body composition, and carbohydrate and lipid metabolism of obese young men Am J Clin Nutr 1979 32: 1776–1787.
Acknowledgements
This project was supported by a grant from the American Heart Association #9507837S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Donnelly, J., Jacobsen, D., Snyder Heelan, K. et al. The effects of 18 months of intermittent vs continuous exercise on aerobic capacity, body weight and composition, and metabolic fitness in previously sedentary, moderately obese females. Int J Obes 24, 566–572 (2000). https://doi.org/10.1038/sj.ijo.0801198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0801198
Keywords
This article is cited by
-
Hepatic Runx1t1 improves body fat index after endurance exercise in obese mice
Scientific Reports (2023)
-
Investigating the practical viability of walk-sharing in improving pedestrian safety
Computational Urban Science (2021)
-
Association of Exercise with Control of Eating and Energy Intake
Current Addiction Reports (2019)
-
The effect of brisk walking on postural stability, bone mineral density, body weight and composition in women over 50 years with a sedentary occupation: a randomized controlled trial
BMC Women's Health (2016)
-
Physical Activity, Energy Intake, and Obesity: The Links Between Exercise and Appetite
Current Obesity Reports (2013)