Abstract
Treatment options for T3N1 stage gastric cancer exhibit regional variation, with optimal approach remaining unclear. We derived our data from the SEER database, using Cox proportional risk regression models for univariate and multivariate analyses of 5-years overall survival (5yOS) and 5-years cancer-specific survival (5yCSS). The results showed that younger age, female, non-white race, highly differentiated histologic grade, non-Signet ring cell adenocarcinoma, low N stage, lesser curvature of the stomach, OP followed by adjuvant C/T with or without RT, partial gastrectomy, C/T and others, Radiation therapy, and Chemotherapy were significantly associated with better 5yOS and 5yCSS. For patients with stage T3N1-3 gastric cancer, multimodal treatment regimens demonstrate superior survival outcomes compared to surgery or radiotherapy alone. Among them, OP followed by adjuvant C/T with or without RT emerges as particularly efficacious, potentially offering enhanced benefits for non-Asian populations.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is not only the fifth most prevalent cancer in the world, with more than 1 million new cases each year, but also the fourth leading cause of cancer death, according to the latest global cancer burden data for 2020 released by the International Agency for Research on Cancer (IARC) of the World Health Organization1,2. With the advancement of diagnosis and treatment technology, the 5-years survival rate of early gastric cancer patients has exceeded 90%3. Nonetheless, survival outcomes hinge significantly on tumor staging, with locally advanced gastric cancer prognosis remaining grim due to its aggressive nature and swift progression4. Moreover, variations in tumor biological characteristics, patient demographics, and treatment strategies contribute to significant disparities in the 5-years survival rates of gastric cancer patients across different regions5,6,7.
Strategies for the treatment of locally advanced gastric cancer aimed at improving patient survival and requiring expertise from multiple disciplines, and multidisciplinary treatment decisions are made after a comprehensive evaluation by specialists in oncology, surgery, radiotherapy, imaging, and pathology4,7. Several regional treatment guidelines recommend radical gastrectomy combined with neoadjuvant chemotherapy, perioperative chemotherapy, and postoperative adjuvant chemotherapy (with or without radiotherapy) for patients with locally advanced gastric cancer8,9,10. Recent studies have revealed the possibility of modulating cellular composition and function within the tumor microenvironment to influence gastric cancer growth, metastasis, and survival outcomes. Nonetheless, the limited overall therapeutic efficacy, significant inter-individual variability in response, and safety concerns associated with immunotherapy have impeded its widespread adoption and utilization11,12,13. Currently, there are numerous treatment options for stage T3N1-3 gastric cancer, however, decisions on treatment regimens vary somewhat by region, and the optimal treatment remains unclear14,15,16. Through a retrospective analysis of the SEER database 17 to compare the overall and specific survival rates of different treatment modalities for T3N1-3 gastric cancer, thereby discerning the optimal therapeutic approach for T3N1-3 gastric cancer.
Methods
Our research materials were derived from demographics, tumor characteristics, treatment regimens, and outcome data for patients with gastric cancer diagnosed between 2010 and 2015 from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database. Study data were extracted from the SEER 17 registry database using SEER*Stat software (version 8.4.2). The study was conducted using de-identified medical information data from the SEER database and therefore no ethics committee approval and informed consent from patients was required.
Study population
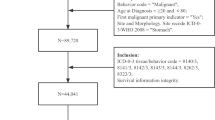
Participants meeting the following criteria were included in this study: (1) All patients were diagnosed with gastric cancer in 2010–2015. (2) Age at diagnosis > 18 years. (3) Compliance with AJCC Cancer Staging Manual 7th edition TNM staging of T3, N1-3, M0. (4) No other type of cancer diagnosed in a lifetime. Participants were excluded if (1) Survival months were 0 or unknown. (2) Neither surgical treatment nor radiotherapy was received. (3) Not histologically confirmed. (4) Gastric cancer is not the first malignant tumor.
Data extraction
Extracted data included sex, age at diagnosis, race record, marital status at diagnosis, primary site, ICD-O-3 histology code and behavior, CS tumor size, CS lymph nodes, Regional nodes positive, Regional nodes examined, pathologic grade, derived AJCC T and N stages, radiation sequence with surgery, systemic therapy sequence with surgery, RX Summary-Surgery Primary Site, radiation recode, chemotherapy recode, survival months, vital status recode, COD to site recode, SEER cause-specific death classification.
Statistical analysis
Statistical analysis and plotting were performed using R language (version 4.3.1). Continuous variables were converted to categorical variables, and the chi-square test was used to compare the clinicopathologic characteristics of gastric cancer patients with different N-stages. Overall survival (OS) was defined as the time from the month of diagnosis to death from any cause or the last follow-up. Cancer-specific survival (CSS) was defined as cancer death, that is, the time from the month of diagnosis to death from gastric cancer or the last follow-up visit. Survival analysis was performed by plotting survival curves using the Kaplan–Meier method, and differences in CSS and OS between variables were compared by the log-rank test, and P values were determined. Univariate and multivariate analyses of CSS and OS were conducted using the Cox proportional risk regression model. P < 0.05 indicated that the difference was statistically significant.
Results
Ultimately, 3915 patients with T3N1-3M0 gastric cancer were enrolled in this study, with the study population screening process depicted in Fig. 1. Among them, 2013 patients were in the T3N1M0 stage, 1103 with T3N2M0 stage, and 799 with T3N3M0 stage (refer to Table 1 for details). The general characteristics of the population of 3915 gastric cancer patients included in this study were skewed toward ≥ 60 years of age (N1: 1452 [72.13%]; N2:755 [68.45%]; N3: 525 [65.71%]), male (N1: 1479 [73.47%]; N2: 810 [73.44%]; N3: 554 [69.34%]), white (N1: 1576 [78.29%]; N2: 804 [72.89%]; N3: 530 [66.33%]), and married (N1: 1247 [61.95%]; N2: 708 [64.19%]; N3: 513 [64.21%]).
The oncological characteristics of the gastric cancer patients in this study were as follows. In terms of tumor location, Cardia (N1: 59.56%; N2: 44.88%; N3: 30.29%) and Gastric antrum (N1: 13.21%; N2: 17.14%; N3: 21.90%) were predominant. Tumor histology was dominated by Adenocarcinoma, NOS (N1: 60.06%; N2: 53.67%; N3: 44.31%) and Signet ring cell adenocarcinoma (N1: 13.26%; N2: 17.77%; N3: 21.53%). Histologic grading mainly consisted of poorly differentiated (N1: 56.23%; N2: 64.73%; N3: 75.47%) and moderately differentiated (N1: 28.51%; N2: 24.12%; N3: 16.02%). Notably, the proportion of tumors with a size ≥ 5cm increased progressively with advancing N stage, correlating with a heightened risk of lymph node metastasis.
Variations in treatment selections among gastric cancer patients across different N stages were observed in this study. Predominantly, patients in N1 stage favored adjuvant chemotherapy (C/T) and others (45.31%), while a notable proportion opted for concurrent chemoradiotherapy (CCRT) combined with operation (OP) (21.11%). A total of 31.37% of patients with stage N2 gastric cancer opted to receive C/T and others, whereas 25.39% received OP followed by adjuvant C/T with or without radiotherapy (RT). The majority of N3-stage patients received OP followed by adjuvant C/T with or without RT (44.06%) or OP alone (24.28%). The likelihood of surgical intervention increased with escalating N stage, with partial gastrectomy emerging as the most frequently chosen surgical modality (N1: 42.62%; N2: 52.04%; N3: 56.82%). Chemotherapy was administered to the majority of patients (N1: 81.57%; N2: 79.33%; N3: 72.59%), with minimal disparity observed in the proportion of patients with T3N1-3M0 gastric cancer receiving radiotherapy compared to those who did not.
There were significant differences in the 1-, 3-, and 5-years overall survival rates between N1-N3 stages. The 1yOS was above 60% in all N1-N3 patients, but the 5yOS was lower and exhibited considerable variability (N1: 31.2%; N2: 25.9%; N3: 17.6%). The 5yOS and 5yCSS of the multidisciplinary treatment modalities were significantly better than those of OP alone and C/T and other treatment modalities.
A comparison of 5yOS and 5yCSS in T3N1-3 gastric cancer under different treatment regimens was performed (see Supplementary Material for details). Treatment categories included OP alone (group 1), OP followed by adjuvant C/T with or without RT (group 2), CRRT followed by OP (group 3), neoadjuvant C/T followed by OP and adjuvant C/T with or without RT (group 4), and C/T in conjunction with other modalities (group 5). There were significant differences in 5yOS and 5yCSS under different treatment regimens in patients with N1-3 stages of gastric cancer. Figures 2 and 3 displayed the 5yOS and 5yCSS survival curves based on different treatment regimens for gastric cancer patients with different N-stages. Notably, 5yOS was suboptimal, falling below 50% for N1-3 stages of gastric cancer. Overall, the 5yOS with OP only or C/T and others was lower than that of the other three treatment groups, in which the 5yOS of T3N3 gastric cancer treated with OP only was only 5%. The 5yOS was the highest for all stages of gastric cancer treated with OP combined with adjuvant C/T with or without RT, in which the 5yOS for T3N1 stage gastric cancer patients treated with neoadjuvant C/T followed by OP and adjuvant C/T with or without RT was comparable to that of OP followed by adjuvant C/T with or without RT. In contrast, the 5yCSS with OP only was comparable to multidisciplinary combination therapy in patients with T3N1 stage gastric cancer, and the difference between 5yCSS (46.6%) and 5yOS (33.6%) was greater with OP only. We speculated that it might be related to the number of regional lymph node metastases. The conclusions of 5yCSS and 5yOS for gastric cancer with different N-stages were consistent, with the rank sum test indicating significant differences between different treatments (P < 0.05).
A comparison of 5yOS and 5yCSS in T3N1-3 gastric cancer under different surgical therapies was performed (see Supplementary Material for details). Notably, patients who did not undergo surgery exhibited 5yCSS rates below 20%, underscoring the importance of surgical intervention. Partial gastrectomy yielded improved survival outcomes; however, notable discrepancies were observed among different N-staging groups (N1: 52.6%; N2: 37.9%; N3: 25.2%). There were significant differences between different surgical approaches (P < 0.05). The 5yOS and 5yCSS survival curves for different surgical approaches are shown in Supplementary Material.
Univariate and multivariate analyses using Cox proportional risk regression models indicated that younger, female, non-white race, highly differentiated histologic grade, non-Signet ring cell adenocarcinoma, low N stage, lesser curvature of the stomach, OP + adjuvant C/T ± RT, partial gastrectomy, C/T and others, Radiation therapy, and Chemotherapy were significantly associated with better 5yOS and CSS. However, previously married patients with gastric cancer have a poorer prognosis. (See Table 2 for details).
Discussion
Gastric cancer is the fourth largest cancer threatening human health and survival, and with the improvement of diagnosis and treatment, its 5-years survival rate is increasing. Notably, adequate surgery stands as the sole curative treatment known thus far. However, owing to the aggressive nature of locally advanced gastric cancer, even after complete surgical resection, the incidence of postoperative recurrence and complications remains high, leading to a poor prognosis17. A multicenter phase III clinical trial in France demonstrated that the 5-years survival rate of surgical treatment alone is less than 25%, highlighting the imperative for combining other treatment modalities to enhance gastric cancer survival rates18. Conversely, adjuvant radiotherapy alone does not confer survival benefits in multidisciplinary comprehensive treatment approaches for gastric cancer14. Similar to the findings of existing studies, our retrospective analysis of different treatment modalities in patients with T3N1-3 gastric cancer found that multidisciplinary combination therapy (such as OP followed by adjuvant C/T with or without RT, CRRT followed by OP, and neoadjuvant C/T followed by OP and adjuvant C/T with or without RT) compared with OP and C/T and others alone provided a significant benefit for T3N1-3 gastric cancer patients with better 5-years survival. Among them, OP followed by adjuvant C/T with or without RT had the highest 5-years survival rate.
MAGIC and ACCORD-07, two randomized clinical trial studies, demonstrated that the perioperative chemotherapy group and the neoadjuvant chemotherapy group improved overall survival and progression-free survival (PFS) in patients with resectable adenocarcinoma of the stomach, lower esophagus, or gastroesophageal junction compared with the surgical treatment group, without increasing the rate of postoperative complications and the number of deaths within the first 30 days after surgery18,19.
Our study findings reveal that the OP followed by adjuvant C/T with or without RT treatment approach is associated with the highest 5-years survival rates. Similar conclusions were drawn from several trials. Both the ACTS-GC and CLASSIC trials demonstrated that postoperative chemotherapy improves overall survival (OS) and recurrence-free survival (RFS) in patients with gastric cancer after D2 gastrectomy compared with surgical treatment alone20,21. A meta-analysis enrolling 3838 patients after gastrectomy for gastric cancer revealed that postoperative adjuvant chemotherapy based on a fluorouracil regimen improved the 5yOS of gastric cancer compared to surgery alone22. The Intergroup 0116 (INT-0116) clinical trial demonstrated that the addition of adjuvant radiotherapy to chemotherapy based on fluorouracil and folinic acid significantly improved OS and RFS, and significantly reduced overall recurrence and local recurrence23. The ARTIST clinical trial illustrated the role of adjuvant radiochemotherapy in D2-resected gastric cancer patients, directly comparing it with fluoropyrimidine-platinum combination adjuvant chemotherapy. It was ultimately concluded that adjuvant chemotherapy and radiotherapy have similar disease-free survival (DFS) and OS in D2 resected GC and are equally beneficial in preventing recurrence. However, radiochemotherapy significantly improved DFS in patients with lymph node-positive disease and intestinal-type GC24.
In light of the above clinical trials and meta-analysis, a multidisciplinary treatment paradigm using surgery combined with CT or RT has become the standard treatment option for locally advanced gastric cancer. However, there is no single regimen that is universally accepted as the standard for multimodal treatment, and geographic disparities persist in the utilization of adjuvant chemotherapy, postoperative (adjuvant) radiotherapy, or perioperative chemotherapy (neoadjuvant). For example, in Asia adjuvant chemotherapy significantly improves the survival prognosis of patients with gastric cancer, whereas Western centers failed to achieve similar clinical outcomes. A joint study from the European Organization for Research and Treatment of Cancer (EORTC) Institutes and the International Cooperation on Cancer (ICCG) Institutes compared the efficacy of combined 5-fluorouracil with adriamycin or epirubicin and methotrexate with leucovorin rescue (FAMTX or FEMTX) with surgical treatment alone in terms of disease-free survival (DFS) and overall survival (OS). It was found that there was no significant difference in DFS and OS between the two groups, however, the survival rate was higher in the EORTC group compared to the ICCG group, which may be related to the more thorough surgery performed in the EORTC study25. Similarly, the results of two Italian phases III clinical trials, the GOIRC study and the GOIM 9602 study, showed no difference in OS or DFS between the adjuvant chemotherapy group (PELF regimen or ELFE regimen) and the surgery alone group26,27. Consistent with these findings, by comparing the 5yCSS of T3N1-3 gastric cancer patients treated with surgery alone versus adjuvant chemotherapy, we found that for patients with stage T3N1 surgery alone possessed a better 5yCSS, whereas for patients with stages T3N2 and T3N3 the 5yCSS between adjuvant chemotherapy and surgery alone was similar.
Limitation
Our study has certain limitations. First, the geographical limitation of our study may not be representative. It cannot represent the efficacy of treatment programs for gastric cancer patients in other regions. Second, the use of a retrospective dataset for our study may cause bias in the results. Third, we were unable to obtain more detailed information about each treatment for each patient, such as the duration of systemic therapy, specific chemotherapeutic agents and dosages, time intervals between surgery and radiotherapy or chemotherapy, targeted therapies, immunotherapy, chemotherapeutic toxicity, and changes in quality of life. Fourth, there was a lack of information about the patient's gastrointestinal and hematologic history, physiologic function, and other influences that are most likely to change the patient's treatment plan and prognosis. Finally, the small sample size did not allow for more statistically significant results. Existing studies have shown some variation in the choice of treatment regimen across regions, and broader and more in-depth studies are needed in the future to determine the optimal treatment regimen and the survival benefit it provides. However, the SEER database covers approximately 30% of the U.S. population, and the results of future clinical studies will become increasingly compelling as the inclusion population continues to expand.
Conclusion
A multimodal treatment regimen has a better survival prognosis than surgery and radiotherapy alone for patients with stage T3N1-3 gastric cancer. In particular, OP followed by adjuvant C/T with or without RT had the highest survival rate. This treatment regimen may be more suitable for non-Asian populations. In the future, multiregional, high-quality, large-scale, and long-term follow-up studies are needed to clarify the standard treatment strategy for patients with stage T3N1-3 gastric cancer.
Data availability
The dataset used in this study was obtained from the SEER 17 registry database of the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) program. For instructions on accessing these data, please visit https://seer.cancer.gov/.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73(1), 17–48 (2023).
Necula, L. et al. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 25(17), 2029–2044 (2019).
Goetze, O. T., Al-Batran, S. E., Chevallay, M. & Mönig, S. P. Multimodal treatment in locally advanced gastric cancer. Updates Surg. 70(2), 173–179 (2018).
Torre, L. A., Siegel, R. L., Ward, E. M. & Jemal, A. Global cancer incidence and mortality rates and trends: An update. Cancer Epidemiol. Biomark. Prev. 25(1), 16–27 (2016).
The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020, 5(1):42–54.
Mueller, J. L. et al. Nature versus nurture: The impact of nativity and site of treatment on survival for gastric cancer. Gastr. Cancer 22(3), 446–455 (2019).
Ajani, J. A. et al. Gastric cancer, Version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 20(2), 167–192 (2022).
Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26(1):1–25.
Lordick, F. et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33(10), 1005–1020 (2022).
Rihawi, K. et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: Novel translational implications. Int. J. Mol. Sci. 22(8), 3805 (2021).
Mollica, V. et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: The MOUSEION-06 study. Clin. Exp. Med. 23(8), 5039–5049 (2023).
Rizzo, A. et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: The mouseion-05 study. Cancer Immunol. Immunother. 72(6), 1381–1394 (2023).
Merrett, N. D. Multimodality treatment of potentially curative gastric cancer: Geographical variations and future prospects. World J. Gastroenterol. 20(36), 12892–12899 (2014).
Davis, P. A. & Sano, T. The difference in gastric cancer between Japan, USA and Europe: What are the facts? What are the suggestions?. Crit. Rev. Oncol. Hematol. 40(1), 77–94 (2001).
Tokunaga, M. et al. Perioperative chemotherapy for locally advanced gastric cancer in Japan: Current and future perspectives. Surg. Today 50(1), 30–37 (2020).
Van Cutsem, E., Sagaert, X., Topal, B., Haustermans, K. & Prenen, H. Gastric cancer. Lancet 388(10060), 2654–2664 (2016).
Ychou, M. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 29(13), 1715–1721 (2011).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355(1), 11–20 (2006).
Sasako, M. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 29(33), 4387–4393 (2011).
Bang, Y. J. et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 379(9813), 315–321 (2012).
Paoletti, X. et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. Jama 303(17), 1729–1737 (2010).
Smalley, S. R. et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin Oncol. 30(19), 2327–2333 (2012).
Park, S. H. et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J. Clin. Oncol. 33(28), 3130–3136 (2015).
Nitti, D. et al. Randomized phase III trials of adjuvant FAMTX or FEMTX compared with surgery alone in resected gastric cancer: A combined analysis of the EORTC GI Group and the ICCG. Ann Oncol. 17(2), 262–269 (2006).
Di Costanzo, F. et al. Adjuvant chemotherapy in completely resected gastric cancer: A randomized phase III trial conducted by GOIRC. J. Natl. Cancer Inst. 100(6), 388–398 (2008).
De Vita, F. et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann. Oncol. 18(8), 1354–1358 (2007).
Author information
Authors and Affiliations
Contributions
Study concepts: Q.T., Y.T.; study design: Q.T., Y.T.; data acquisition: Y.T., S.L.; Quality control of data and algorithms: Y.T., S.L.; Data analysis and interpretation: Y.T., S.L., S.T., H.C., M.H.; Statistical analysis: S.T., H.C., M.H.; Manuscript preparation: Y.T. and S.L.; manuscript editing: Y.T. and S.T.; Manuscript review: Q.T.; final approval of manuscript: all authors; accountable for all aspects of the work: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, Y., Liu, S., Tao, S. et al. Comparison of different treatment strategies for T3N1-3 stage gastric cancer based on the SEER database. Sci Rep 14, 11210 (2024). https://doi.org/10.1038/s41598-024-61904-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61904-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.