Abstract
Electrochemical carbon dioxide/carbon monoxide reduction reaction offers a promising route to synthesize fuels and value-added chemicals, unfortunately their activities and selectivities remain unsatisfactory. Here, we present a general surface molecular tuning strategy by modifying Cu2O with a molecular pyridine-derivative. The surface modified Cu2O nanocubes by 4-mercaptopyridine display a high Faradaic efficiency of greater than 60% in electrochemical carbon monoxide reduction reaction to acetate with a current density as large as 380 mA/cm2 in a liquid electrolyte flow cell. In-situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy reveals stronger *CO signal with bridge configuration and stronger *OCCHO signal over modified Cu2O nanocubes by 4-mercaptopyridine than unmodified Cu2O nanocubes during electrochemical CO reduction. Density function theory calculations disclose that local molecular tuning can effectively regulate the electronic structure of copper catalyst, enhancing *CO and *CHO intermediates adsorption by the stabilization effect through hydrogen bonding, which can greatly promote asymmetric *CO-*CHO coupling in electrochemical carbon monoxide reduction reaction.
Similar content being viewed by others
Introduction
Acetate is an important chemical to manufacture food additives, solvents, medicine, etc1,2. The global demand for acetate was 17.3 million tons in 2019 and is predicted to reach 24.5 million tons by 20253. Nowadays, methanol carbonylation still serves as the main method to produce acetate, which is energy-consuming and environmentally unfriendly4,5,6,7. On the other hand, electrochemical carbon dioxide/carbon monoxide reduction reaction (CO2RR/CORR) powered by renewable electricity provides a greener approach to make acetate8,9,10,11,12. However, the selectivity and the partial current density towards the targeted acetate via CO2RR/CORR are still unsatisfactory, and the reaction pathway for CO2RR/CORR to acetate remains ambiguous.
Cu-based electrocatalysts have been intensively studied in CO2RR/CORR to make C2+ fuels/chemicals, but they still suffer from poor selectivity13. Various strategies have been applied to tackle the poor selectivity challenge, including alloying, facet engineering, surface regulation, etc14,15,16,17,18,19,20,21,22,23. Among the different approaches, modifying Cu surface with certain small molecule was proposed as a promising route to modulate the reaction pathways for generation of C2+ fuels/chemicals24,25.
Molecules exhibited multiple-functions on CO2RR determined by the structure of molecules26,27. Molecular decoration is able to adjust the strength of intermediates adsorption and selectively stabilize certain intermediates through hydrogen bonding or enhance the adsorption of certain intermediates via confinement effect or regulating the catalyst’s electronic structure to boost the catalytic performance28,29,30. However, insufficient understanding of the underlaying principle of small molecule modification on electrochemical CO2RR/CORR remains a major factor hindering the development of this strategy.
In this work, we presented a universal strategy to modify Cu-based catalysts with 4-mercaptopyridine (pyS), including commercial Cu, Cu2O, CuO as well as the as-prepared Cu2O nanocubes, which could dramatically enhance the catalytic performance for electrochemical CORR to produce acetate. The molecular tuning induced by 4-mercaptopyridine over Cu surface enhanced the adsorption of reaction intermediates in CORR, improved the hydrogenation of *CO, facilitated asymmetric *CO-*CHO coupling and thus stimulated the CO-to-acetate conversion. The surface modified Cu2O nanocubes by 4-mercaptopyridine (Cu2O-pyS) exhibited a high acetate FE of >60% at a total current density of 380 mA cm−1 in a liquid electrolyte flow cell, whose performance remained almost unchanged at 380 mA/cm2 with an acetate FE over 60% for 100 h in a flow cell. The impressive CORR performance of Cu2O-pyS originated from the boosted asymmetric C-C coupling induced by 4-mercaptopyridine modification, validated by in-situ ATR-SEIRAS measurements and DFT calculations.
Results
Catalyst preparation and CORR performance
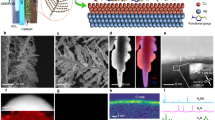
Cu2O nanocubes were synthesized and further functionalized with 4-mercaptopyridine. Cu2O nanocubes with a mean size of ~50 nm was synthesized by a facile solution method and then modified with 4-mercaptopyridine under ultrasonication (Supplementary Fig. 1a). X-ray diffraction (XRD) of Cu2O-pyS displays peaks at 29.6°, 36.4°, and 43.4°, which can be assigned to Cu2O (110), Cu2O (111) and Cu2O (200), respectively (JCPDS file NO. 05-0667, Supplementary Fig. 1b). Compared to unmodified Cu2O nanocubes, the infrared spectrum of Cu2O-pyS exhibits several characteristic peaks. The peaks at 788 cm−1, 1460 cm−1, and 1598 cm−1 can be assigned to the stretching vibration of C=S, C=C and pyridine ring, respectively, demonstrating the successful introduction of 4-mercaptopyridine onto Cu2O nanocubes (Supplementary Fig. 1c). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Supplementary Figs. 2a, b and 3a, b) indicate that the Cu2O-pyS maintains the well-defined nanocube morphology after 4-mercaptopyridine modification. High-resolution energy-dispersive X-ray spectroscopy (EDS) line scan and mapping over Cu2O-pyS show homogeneous distribution of Cu, O, N, and S elements, suggesting successful attachment of 4-mercaptopyridine on the surface of Cu2O nanocubes (Supplementary Figs. 2d and 3d). The clear lattice fringe of 0.213 nm observed on Cu2O-pyS can be assigned to Cu2O (200) (Supplementary Figs. 2c and 3e). The electronic structure of Cu2O-pyS was probed by X-ray photoelectron spectroscopy (XPS) and it was found that the binding energy of Cu in Cu2O-pyS was shifted towards higher binding energies after 4-mercaptopyridine modification (Supplementary Fig. 4a). High-resolution N 1s and S 2p XPS spectra of Cu2O-pyS display clear peaks originating from 4-mercaptopyridine. The high-resolution N 1s XPS spectrum can be deconvoluted into pyridine N (397.5 eV) and N with different degrees of H acceptance, respectively31. The high-resolution S 2p XPS spectrum displays a peak at 162 eV (Supplementary Fig. 4b), indicating the formation of Cu-S bond32. The presence of thiolate and the absence of metal-N bond corroborate that 4-mercaptopyridine is linked on Cu2O via the Cu-S bond.
The CORR was evaluated in a flow-cell electrolyzer to overcome the poor solubility of CO and the sluggish CO mass transport in aqueous solution (Supplementary Figs. 5 and 6)33,34,35. The electrochemical performance of Cu2O-pyS was also evaluated. As presented in Fig. 1a & Supplementary Fig. 7, CO could be electrochemically reduced on Cu2O-pyS to acetate, exhibiting a total current density of 380 mA/cm2 with an acetate FE (FEacetate) as high as 62% at −0.85 V versus reversible hydrogen electrode (RHE). A higher FEacetate of 70% could be realized at −0.65 V vs. RHE over Cu2O-pyS. As compared to Cu2O-pyS, the FE and partial current density towards acetate over unmodified Cu2O nanocubes are much lower, indicating the critical role of 4-mercaptopyridine played in the CORR (the different electrochemically active surface area (ECSA) contribution could be excluded as shown in Supplementary Fig. 8). To verify the universality of the pyS-modification strategy, commercial Cu, Cu2O, and CuO were also modified with 4-mercaptopyridine, denoted as c-Cu-pyS, c-Cu2O-pyS, and c-CuO-pyS, respectively. After decoration of 4-mercaptopyridine, obvious enhancement in acetate production (selectivity and partial current density) could be observed for all commercial samples (Fig. 1b–d and Supplementary Fig. 9), confirming the generality of the pyS-modification approach. Besides activity and selectivity, stability is also crucial in CORR. The CORR stability of Cu2O-pyS was evaluated in a 9 cm2 flow cell. As displayed in Fig. 2a, the Cu2O-pyS could be operated continuously at a current density of 380 mA/cm2 and a FEacetate over 60% for 100 h with no noticeable activity decay, outperforming most of the reported state-of-the-art CORR electrocatalysts. It is worth mentioning that 2.08 mol of acetate, derived from superposition of current and Faradaic efficiency, could be produced in 100 h. SEM, TEM, and EDS measurements disclosed that the morphology and size of Cu2O nanocubes and Cu2O-pyS after CORR were changed (Supplementary Figs. 10 and 11). Both of Cu2O nanocubes and Cu2O-pyS were reduced to Cu under applied cathodic potentials during CORR (Supplementary Fig. 10a, b). Moreover, N and S could still be observed over Cu2O-pyS after the CORR, indicating that 4-mercaptopyridine was stable under the applied cathodic potential (Supplementary Fig. 11c, d).
a CORR stability test of Cu2O-pyS. Inset is the schematic illustration showing the flow cell. b Quasi in-situ time-dependent Cu LMM X-ray-excited Auger electron spectra of Cu2O-pyS collected at −0.65 V vs. RHE. Quasi in-situ time-dependent high-resolution N 1s XPS spectra (c), and S 2p XPS spectra (d) collected at −0.65 V vs. RHE. e In-situ ATR-SEIRAS spectra from 1000 cm−1 to 2300 cm−1 recorded over Cu2O-pyS in CO-saturated 0.1 mol/L KOH solution at −0.65 V vs. RHE.
Stability evaluation for CORR
To elucidate the structural evolution of Cu2O-pyS during CORR, in-situ Raman spectroscopy was conducted. Raman peaks at 146 and 219 cm−1 were observed over Cu2O nanocubes and Cu2O-pyS at the open circuit potential (OCP) (Supplementary Figs. 12 and 13), which are characteristic Raman peaks of Cu2O36. With increase in the applied cathodic potential, the intensity of the Cu2O characteristic Raman peaks decreased and finally disappeared, suggesting that Cu2O was reduced to Cu. More importantly, the Raman peaks at 689 and 783 cm−1, which could be assigned to Cu-S vibrational mode, resulting from 4-mercaptopyridine remained stable even at the applied potential of −1.0 V vs. RHE, which is already the CORR working potential for the catalyst, illustrating the excellent affinity of 4-mercaptopyridine over the copper surface. Additionally, Cu-C vibration (~300 cm−1) could also be observed in the in-situ Raman spectra (as shown in Supplementary Fig. 13), resulting from the reactive intermediates in the CORR, which will be discussed later. Quasi in-situ Auger electron spectroscopy (AES) was performed to probe the valance state evolution of Cu during the reaction, where Cu2O nanocubes was rapidly reduced to Cu0 after 0.5 h (Fig. 2b). Similar results were also observed in commercial Cu2O sample (Supplementary Fig. 14). Quasi in-situ time-dependent high-resolution N 1 s and S 2p XPS spectra also confirmed that 4-mercaptopyridine could stay on the surface of Cu2O catalyst at all times (Fig. 2c, d and Supplementary Fig. 15). To further elucidate the structural stability of Cu2O-pyS, in-situ time-dependent ATR-SEIRAS measurement was carried out (Supplementary Fig. 16). DFT calculation was performed to identify the positions of the vibrational peaks (Supplementary Fig. 25). Compared to Cu2O nanocube catalyst, a ATR-SEIRAS peak at around 1,600 cm−1 could be observed on Cu2O-pyS, which can be assigned to pyridine ring skeletal vibration, revealing the successful attachment of 4-mercaptopyridine onto the Cu2O surface. As shown in Fig. 2e, the pyridine ring skeletal vibration remained in 24 hours of CORR. Moreover, peaks for *COL, *COB, *CHO, *COCHO, and H2O existed throughout the reaction, indicating that 4-mercaptopyridine was stable over the surface of the catalyst (Fig. 2e). X-ray absorption spectroscopy (XAS) was further conducted to disclose the structural and electronic evolution of Cu element. Figure 3a, c revealed that Cu2O continued to be reduced in CORR. First derivative of X-ray absorption near edge structure (XANES) was analyzed to investigate the reduction of Cu2O. The results showed that Cu2O was converted into Cu under the applied cathodic potential, and the transformation was accelerated after decoration of 4-mercaptopyridine (Fig. 3b, d). The k3-weighted extended X-ray absorption fine structure (EXAFS) and the corresponding Fourier transform of EXAFS for Cu2O nanocubes and Cu2O-pyS recorded during CORR are shown in Supplementary Fig. 17. It is worth mentioning that Cu0 remained after its appearance, suggesting that Cu2O (-pyS) derived Cu (-pyS) was the real catalyst for CORR to acetate.
a In-situ Cu K-edge XANES spectra of Cu2O nanocubes measured in CO-saturated KOH solution at −0.65 V vs. RHE. b The corresponding 1st derivative of XANES at −0.65 V vs. RHE. c In-situ Cu K-edge XANES spectra of Cu2O-pyS measured in CO-saturated KOH solution at −0.65 V vs. RHE. d The corresponding 1st derivative of XANES at −0.65 V vs. RHE.
Mechanistic insights for CORR
To further shed light on the underlying role of pyS decoration on CORR, in-situ ATR-SEIRAS measurements under different applied cathodic potentials were performed to probe the reaction intermediates and the reaction pathway. Supplementary Figs. 18 and 19 show the ATR-SEIRAS spectra recorded over Cu2O nanocubes. Compared to Cu2O, the water peak at ~1625 cm−1 is less pronounced on Cu2O-pyS, suggesting that the presence of pyS can help to reduce water adsorption on the catalyst’s surface, which may lower the undesired hydrogen evolution reaction (HER) over Cu2O. When the applied cathodic potential increased to −0.2 V vs. RHE, several ATR-SEIRAS peaks started to appear over Cu2O-pyS. Two types of *CO peaks could be found in the ATR-SEIRAS spectra, one at 2080 cm−1, which can be assigned to the linearly bound *CO (COL); the other at 1800 cm−1, which can be ascribed to the bridge-bound *CO (Fig. 4a, b). Both *COL and *COB are reactive species in CORR towards acetate formation (Supplementary Figs. 20 and 21). The ratio of *COB/*COL over Cu2O-pyS is much larger than that over Cu2O, implying that 4-mercaptopyridine could influence *CO adsorption configuration (Fig. 4c and Supplementary Figs. 20 and 21). Between the two types of *CO, *COB is much easier to participate in the hydrogenation reaction due to its weak C=O bond, in contrast to the strong C≡O bond in *COL37. In addition, *CHO signal at 1,185 cm−1 and *OCCHO signal at 1,040 cm−1 could also be clearly identified over Cu2O-pyS, which was further supported by the isotope labeling experiments (Supplementary Fig. 22). Previous reports have shown that *OCCHO plays as an important intermediate in CO2RR/CORR to produce acetate38. Figure 4d shows that the ATR-SEIRAS peak ratio of *OCCHO/*CHO over Cu2O-pyS is at least one order of magnitude larger than that over Cu2O, justifying the boosted CORR to acetate over Cu2O-pyS. The role of *OCCHO for CO-to-acetate conversion can be further confirmed by the observation that the *OCCHO coverage increased with applied cathodic potential. Two C-C coupling pathways were usually proposed in CORR to produce C2+ products (Fig. 4e): the asymmetric *CO-*CHO coupling for acetate formation and the symmetric *CO-*CO or *CHO-*CHO coupling for ethanol and ethylene formation. The detected *OCCHO intermediate can only exist in the pathway of asymmetric *CO-*CHO coupling, explaining the excellent activity and selectivity of CORR to acetate over Cu2O-pyS. Similar phenomenon could also be observed in the case of commercial Cu2O and the corresponding c-Cu2O-pyS, highlighting the importance of 4-mercaptopyridine functionalization (Supplementary Fig. 26). Therefore, 4-mercaptopyridine decoration would be a general strategy to promote CO-to-acetate conversion via boosting asymmetric C-C coupling.
In-situ ATR-SEIRAS spectra recorded in a potential window from 0 to −1.0 V vs. RHE over Cu2O nanocubes (a) and Cu2O-pyS (b) in 0.1 M KOH. c Calculated ratio of *COB/*COL over Cu2O nanocubes and Cu2O-pyS. d Calculated ratio of *OCCHO/*CHO over Cu2O nanocubes and Cu2O-pyS. e Schematic illustration showing the symmetric C-C coupling and asymmetric C-C coupling over Cu2O nanocubes and Cu2O-pyS. Error bars in c and d represent s.d. for each data point (n = 3 independent experiments), and points are average values.
Theoretical understanding
To further investigate the role of 4-mercaptopyridine played in CORR, the 4-mercaptopyridine was intentionally removed from the surface of Cu2O nanocubes by Ar plasma treatment (the successful removal of 4-mercaptopyridine on Cu2O nanocubes was confirmed by the disappearance of N and S signals in the XPS spectra as shown in Supplementary Fig. 23b, c). Note that the valence state of Cu decreased slightly after Ar plasma treatment (Supplementary Fig. 23a). Moreover, SEM and TEM images indicate that the morphology and size of Cu2O nanocubes were maintained after Ar plasma treatment (Supplementary Fig. 23d, e), while the CORR current density and acetate FE were found to decrease sharply after Ar plasma treatment, validating the important role played by 4-mercaptopyridine in CORR to produce acetate (Supplementary Fig. 23f). On the other hand, thiophenol (phS) or 2,6-dimethylthiophenol (pyDMS) was also attached onto the surface of Cu2O nanocubes to explore the contribution of hydrophobicity, C, S or N on CORR (the modified sample is denoted as Cu2O-phS and Cu2O-pyDMS, Supplementary Fig. 24). The acetate FE of CORR over Cu2O-phS significantly decreased, while that over Cu2O-pyDMS remained nearly unchanged as compared to Cu2O-pyS, suggesting that hydrophobicity itself did not play the most significant influence on the performance of CORR and the N atom in 4-mercaptopyridine rather than the S atom governed the CO-to-acetate conversion. To further exclude the contribution of steric effect, a molecule with similar functional group but longer chain (4-(Pyridin-4-yl)thiazole-2-thiol) was used to modify the surface of Cu2O nanocubes, the performance, as shown in Supplementary Fig. 24, was similar to that of Cu2O-pyS and Cu2O-pyDMS, suggesting that the role of steric effect was negligible. To gain a deeper insight into the reaction pathway as well as the reaction mechanism, DFT calculations were carried out. Based on ex-situ XRD, in-situ XPS, in-situ Raman spectroscopy and in-situ X-ray absorption spectroscopy (XAS) characterizations, Cu (111) and 4-mercaptopyridine decorated Cu (111) surface were selected as the calculation models to represent Cu2O nanocubes and Cu2O-pyS, and the calculated free energy diagrams of CORR are displayed in Supplementary Fig. 27. It is obvious that the energy barrier of CO*-CHO* asymmetric coupling was much lower than that of CO*-CO* symmetric coupling over both Cu and Cu-pyS, suggesting that asymmetric coupling was conducive to acetate formation (Fig. 5b, c). Moreover, analyzing coverage of reaction intermediates (including *CO and *CHO) on C-C coupling process (*CO + *CO to *COCO and *CO + *CHO to *COCHO) indicates that the impact of *CHO coverage on ΔG and the corresponding activation barriers is stronger than that of *CO coverage in the *CO + *CHO to *COCHO process (Supplementary Fig. 28). Therefore, *CO hydrogenation to form *CHO is crucial. The rate-determining steps (RDSs) of CORR to form acetate over both Cu2O and Cu2O-pyS are *CO hydrogenation and *CO-*CHO coupling (Fig. 5a). Modifying Cu2O with 4-mercaptopyridine can decrease the energy barrier of *CO hydrogenation and *CO-*CHO coupling from 1.33 to 0.9 eV and 1.16 to 0.81 eV, respectively. Note that starting from *CH2CO, the generation of acetate is exothermic, while the production of ethanol and ethylene are endothermic, revealing the high selectivity towards acetate over Cu-pyS (Supplementary Fig. 27). To explore the origin of the accelerated hydrogenation reaction over Cu2O-pyS, kinetic isotope effect (KIE) of H/D over Cu2O nanocubes and Cu2O-pyS were measured and compared. When H2O was replaced by D2O, the formation of acetate reduced obviously over Cu2O nanocubes with a KIE value of 2.09 and 2.12 at −0.55 V vs. RHE and −0.65 V vs. RHE, respectively, suggesting that the activation and dissociation of water were included in the RDS of CORR over Cu2O. On the contrary, the acetate formation using D2O remained nearly undisturbed over Cu2O-pyS with a KIE value of 1.13 and 1.11 at −0.55 V vs. RHE and −0.65 V vs. RHE, respectively. The value approached 1, suggesting that the dissociation of water was not included in the RDS of CORR over Cu2O-pyS and therefore the hydrogenation of *CO could be expedited over Cu2O-pyS (Supplementary Fig. 29). DFT calculation disclosed that hydrogenation of *COB was more energetically favorable than that of *COL, confirming that hydrogenation steps were accelerated over Cu2O-pyS (Supplementary Fig. 31). Moreover, water dissociation was found to be propelled after the introduction of 4-mercaptopyridine (Supplementary Fig. 32a). N atom in 4-mercaptopyridine assisted to capture one H atom from water, facilitating water dissociation and the subsequent hydrogenation process (Supplementary Fig. 32b). Supplementary Fig. 30 shows charge redistribution occurred between the N atom in 4-mercaptopyridine and the H atom in *CHO and *OCCHO, indicating that 4-mercaptopyridine was beneficial to stabilize *CHO and *OCCHO. Supplementary Fig. 30 presents the differential charge density for various important CORR intermediates over Cu2O and Cu2O-pyS. *CO + *H was found more stable over Cu2O, while *CHO and *OCCHO were more stable over Cu2O-pyS. These observations suggest the role of hydrogen bond in stabilizing *CHO and *OCCHO, which can help to break the Brønsted-Evans-Polanyi (BEP) scaling relationship and boost CO-to-acetate conversion. The projected density of states (PDOS) analysis was then performed to get a better understanding of the reaction mechanism. For *CO + *H step, the Cu d-orbital of Cu2O-pyS exhibits more overlapping with the H s-orbital, indicating favorable hydrogenation of *CO and undesirable HER over Cu2O-pyS (Supplementary Fig. 33a, b). For *CHO, more overlapping between the Cu d-orbital of Cu2O-pyS and the C p-orbital of *CHO was observed and at the same time the N p-orbital of Cu2O-pyS overlapped with the H s-orbital of *CHO, corroborating that *CHO was more stable over Cu2O-pyS (Supplementary Fig. 33c, d). For *OCCHO, enhanced overlapping between the Cu d-orbital of Cu2O-pyS and the C/O p-orbital of *CHO as well as the N p-orbital of Cu2O-pyS and the H s-orbital of *CHO were noticed, justifying the stabilization effect of 4-mercaptopyridine on *OCCHO (Supplementary Fig. 33e, f). The enhanced adsorption to CORR intermediates over Cu2O-pyS can be also supported by its higher d-band center (Supplementary Fig. 34). Based on the above investigations, the reaction pathways of CORR towards various products over Cu2O and Cu2O-pyS are proposed as shown in Supplementary Figs. 35 and 36. To further examine the stability of Cu2O-pyS, Ab initio molecular dynamics (AIMD) simulation was performed. No noticeable geometry change could be observed throughout the AIMD simulation, verifying the thermodynamic and kinetic durability of Cu2O-pyS, matching well with the experiments (Fig. 5d).
a Free energy diagram showing the formation of *CHO and *OCCHO. Insets are the models of transitional states over Cu2O and Cu2O-pyS. b Free energy diagram of symmetric coupling and asymmetric coupling over Cu (111). c Free energy diagram of symmetric coupling and asymmetric coupling over Cu-pyS. d Ab initio molecular dynamics (AIMD) results of Cu2O-pyS. Insets show the side view image of Cu2O-pyS at 0 ps, 3 ps, 5 ps, 8 ps, and 10 ps.
Discussion
To sum up, we have developed a general solution method to prepare 4-mercaptopyridine modified Cu-based catalysts, including commercial Cu, Cu2O, CuO and the synthesized Cu2O nanocubes, which exhibit excellent electrochemical CORR performance to produce acetate. The total current density can reach approximately 380 mA/cm2 with an acetate FE beyond 60% in a flow cell. In-situ ATR-SEIRAS observes stronger *CO signal with bridge configuration and stronger *OCCHO signal over Cu2O-pyS than unmodified Cu2O during CORR. DFT calculations illustrate that local molecular modification can effectively tune the electronic structure of copper catalyst and strengthen *CO and *CHO intermediates adsorption by the stabilization effect through hydrogen bonding, which greatly promotes the asymmetric *CO-*CHO coupling in electrochemical CORR, resulting in an exceptional CO-to-acetate conversion performance.
Methods
Chemicals and materials
Copper (II) chloride (CuCl2), sodium hydroxide (NaOH), ascorbic acid (C6H8O6), 4-mercaptopyridine (C5H5NS), thiophenol (C6H5SH), 2,6-dimethylbenzenethiol (C8H10S) and isopropanol (C3H8O) were purchased from Sigma-Aldrich. All chemicals were used directly without further purification. De-ionized water (DI water) was obtained from Millipore Q water purification system.
Synthesis of Cu2O nanocubes
Cu2O nanocubes were synthesized by an ascorbic acid reduction method at room temperature. Typically, 0.1 mmol of CuCl2 was dissolved in 40 mL of DI water, followed by dropwisely adding 2.5 mL of NaOH aqueous solution (0.2 mol/L). Then the solution was stirred for 5 minutes, followed by dropwisely adding 2.5 mL of ascorbic acid solution (1 mol/L). The mixture was further stirred for another 5 minutes. Finally, the product was harvested by centrifugation and then washed with ethanol thoroughly.
Synthesis of Cu2O-pyS
The as-prepared Cu2O nanocubes were dispersed in N, N-dimethylformamide (DMF) and sonicated for 30 minutes at room temperature, followed by adding a solution containing 4-mercaptopyridine under inert atmosphere. Subsequently, the suspension was sonicated for another 3 hours. Finally, the product was harvested by centrifuge and then washed with DMF and ethanol thoroughly.
Synthesis of c-Cu (Cu2O/CuO)-pyS
The commercial Cu powder (25 nm, Sigma), Cu2O powder and CuO powder were dispersed in N, N-dimethylformamide (DMF) and sonicated for 30 minutes at room temperature, followed by adding a solution containing 4-mercaptopyridine under inert atmosphere. Subsequently, the suspension was sonicated for another 3 hours. Finally, the product was harvested by centrifuge and then washed with DMF and ethanol thoroughly.
Materials characterization
Powder XRD was performed on a Bruker D2 Phaser using Cu Kα radiation with a LYNXEYE detector at 30 kV and 10 mA. The morphological information was examined with field-emission SEM (FESEM, JEOL JSM-6700F). Sub angstrom-resolution high-angle annular dark field scanning transmission electron microscopy (HAADFSTEM) characterization was conducted on a JEOL JEMARM200F STEM with a guaranteed resolution of 0.08 nm. Raman spectra were recorded on a Renishaw INVIA Reflex Raman spectrometer using 514 nm laser as the excitation source. XPS measurements were carried out on a Thermofisher ESCALAB 250Xi photoelectron spectrometer (Thermofisher Scientific) using a monochromatic Al Kα X-ray beam (1,486.6 eV)39.
Electrode fabrication
A catalyst ink solution containing 10 mg of Cu2O-pyS, 0.98 mL of DI water, 0.98 mL of isopropanol and 40 µl of Nafion ionomer solution (5 wt.% in H2O) was mixed and sonicated for at least 3 hours. Then, the ink was deposited onto a carbon paper to achieve a catalyst loading of ~1 mg/cm2. Cu2O electrode was also prepared using the same method. To ensure precise control of the catalytic layer thickness and achieve good uniformity, we employed ultrasonic spraying method to prepare the electrode. This involves two steps: (1) Ultrasonic dispersion to prepare catalyst paste; (2) Atomization and ultrasonic spraying of the catalyst slurry onto a support body, which can be either a gas diffusion layer or proton exchange membrane.
Electrochemical measurements
CORR was performed in 1 M KOH solution in a three-channel flow cell. The anode and the cathode were separated by a hydroxide exchange membrane. Hg/HgO electrode was used as the reference electrode, while NiFeMoB developed by our research group40 was deployed as the anode catalyst. The gas flow rate was 20 sccm. The products of CORR were quantified using a gas chromatography (Agilent 7890) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD) and a high-performance liquid chromatography (Agilent Technologies 1260 Infinity) equipped with a RID detector. Acetate measurements were conducted only in the cathode chamber.
In-situ ATR-SEIRAS measurements
The attenuated total reflectance surface enhanced infrared absorption spectroscopy (ATR-SEIRAS) measurements were performed on a Nicolet iS50 FTIR spectrometer equipped with a MCT detector cooled with liquid nitrogen and PIKE VeeMAX III variable angle ATR sampling accessory.
Quasi in-situ XPS measurements
The X-ray photoelectron spectroscopy (XPS) measurements were performed on a SPECS NAP-XPS interconnected with a glovebox (Vigor Corp) (Supplementary Fig. 15). The CORR were performed in the glovebox, afterwards the sample was transferred to the XPS chamber without air exposure.
In-situ Raman measurements
In-situ Raman spectroscopy measurements were conducted in a custom-designed three-electrode SERS flow cell with a saturated Ag/AgCl electrode as the reference electrode and a graphite rod as the counter electrode in the anode chamber. During the measurements, the electrolyte was constantly purged with CO gas and circulated across the cell using a peristaltic pump. The Raman measurements were performed on a LabRAM HR Evolution microscope (Horiba Jobin Yvon) with a 532 nm laser, a 50× objective, a monochromator (600 grooves/mm grating), and a CCD detector41.
Computational methods
All spin-polarized DFT geometry optimizations were implemented under the description of generalized gradient approximation (GGA) based PBE-D3 functional with VASPsol model in the Vienna ab initio Simulation Package (VASP5.4.4). The 4 × 4 periodic rectangular supercell Cu (111) was built to support the 4-mercaptopyridine. The Brillouin zone was sampled by 3 × 3 × 1 Monkhorst-Pack k-point scheme, and the cutoff was set as 450 eV. The convergence criteria were set as 10−5 eV in energy and 0.01 eV A−1 in force, respectively. A 20 Å vacuum space was added along the perpendicular direction to eliminate the effects of periodic images. The climbing image nudged elastic band (CI-NEB) method was employed with converged forces less than 0.05 eV/Å in VTST package to obtain transition states to derive the reaction free energy barriers42. During structure relaxation, the bottom two layers of copper atoms were immobilized, while all other ions were allowed to move freely to reasonably save computing resources. The canonical ensemble (NPT) ab initio molecular dynamic (AIMD) simulations were carried out in the Anderen thermostat at 298 K for 10 picoseconds (ps) with a time step of 1 femtosecond (fs).
In each of the electroreduction elementary steps, the reaction Gibbs free energy (ΔG) was calculated with consideration of thermal internal energy contribution according to:
where ΔE, ΔEZPE and TΔS are the changes of electronic energy, the zero-point energy, and the temperature-entropy product in each elementary step. ΔGU and ΔGpH equaling to −eU and kBTln10 × pH (here pH = 14) are the contribution of applied electrode potential and pH to ΔG. The entropy of H++e− pair is approximately half of H2 entropy in standard condition.
Data availability
All data are reported in the main text and supplementary materials. Source data are provided with this paper. All relevant data are available from the authors on reasonable request. Source data are provided with this paper.
References
Flexer, V. & Jourdin, L. Purposely designed hierarchical porous electrodes for high rate microbial electrosynthesis of acetate from carbon dioxide. Acc. Chem. Res. 53, 311–321 (2020).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Matias, I. A., Ribeiro, A. P. & Martins, L. M. Selective oxidation of ethane to acetic acid catalyzed by a C-scorpionate iron (II) complex: a homogeneous vs. heterogeneous comparison. Molecules 25, 5642 (2020).
Kalck, P., Le Berre, C. & Serp, P. Recent advances in the methanol carbonylation reaction into acetic acid. Coord. Chem. Rev. 402, 213078 (2020).
Thomas, C. M. & Süss-Fink, G. Ligand effects in the rhodium-catalyzed carbonylation of methanol. Coord. Chem. Rev. 243, 125–142 (2003).
Forster, D. On the mechanism of a rhodium-complex-catalyzed carbonylation of methanol to acetic acid. J. Am. Chem. Soc. 98, 846–848 (1976).
Ni, Y. et al. A green route for methanol carbonylation. Catal. Sci. Technol. 7, 4818–4822 (2017).
Zhang, J., Cai, W., Hu, F. X., Yang, H. & Liu, B. Recent advances in single atom catalysts for the electrochemical carbon dioxide reduction reaction. Chem. Sci. 12, 6800–6819 (2021).
Boutin, E. et al. Aqueous electrochemical reduction of carbon dioxide and carbon monoxide into methanol with cobalt phthalocyanine. Angew. Chem. Int. Ed. 58, 16172–16176 (2019).
Rabiee, H. et al. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review. Energy Environ. Sci. 14, 1959–2008 (2021).
Hori, Y., Murata, A., Kikuchi, K. & Suzuki S. Electrochemical reduction of carbon dioxides to carbon monoxide at a gold electrode in aqueous potassium hydrogen carbonate. J. Chem. Soc. Chem. Commun. 10, 728–729 (1987).
Liu, X. et al. Understanding trends in electrochemical carbon dioxide reduction rates. Nat. Commun. 8, 1–7 (2017).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Zhong, D. et al. Coupling of Cu (100) and (110) facets promotes carbon dioxide conversion to hydrocarbons and alcohols. Angew. Chem. Int. Ed. 60, 4879–4885 (2021).
Kim, Y. et al. Time-resolved observation of C–C coupling intermediates on Cu electrodes for selective electrochemical CO2 reduction. Energy Environ. Sci. 13, 4301–4311 (2020).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Chang, X. et al. C−C coupling is unlikely to be the rate‐determining step in the formation of C2+ products in the copper‐catalyzed electrochemical reduction of CO. Angew. Chem. 134, e202111167 (2022).
Duan, G. Y. et al. Highly efficient electrocatalytic CO2 reduction to C2+ products on a poly (ionic liquid)‐based Cu0–CuI tandem catalyst. Angew. Chem. Int. Ed. 61, e202110657 (2022).
Yang, J. et al. Residual chlorine induced cationic active species on porous Cu electrocatalyst for highly stable electrochemical CO2 reduction to C2+. Angew. Chem. 60, 11487–11493 (2021).
Gao, D. et al. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte‐driven nanostructuring. Angew. Chem. 131, 17203–17209 (2019).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2020).
Li, Y. et al. Electrochemically scrambled nanocrystals are catalytically active for CO2-to-multicarbons. Proc. Natl. Acad. Sci. USA 117, 9194–9201 (2020).
Zhang, G. et al. Efficient CO2 electroreduction on facet-selective copper films with high conversion rate. Nat. Commun. 12, 1–11 (2021).
Xie, M. S. et al. Amino acid modified copper electrodes for the enhanced selective electroreduction of carbon dioxide towards hydrocarbons. Energy Environ. Sci. 9, 1687–1695 (2016).
Creissen, C. E. et al. Molecular inhibition for selective CO2 conversion. Angew. Chem. Int. Ed. 61, e202206279 (2022).
Pankhurst, J. R., Iyengar, P., Loiudice, A., Mensi, M. & Buonsanti, R. Metal–ligand bond strength determines the fate of organic ligands on the catalyst surface during the electrochemical CO2 reduction reaction. Chem. Sci. 11, 9296–9302 (2020).
Li, F. W. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 23, 509–513 (2020).
Tamura, J. et al. Electrochemical reduction of CO2 to ethylene glycol on imidazolium ion-terminated self-assembly monolayer-modified Au electrodes in an aqueous solution. Phys. Chem. Chem. Phys. 17, 26072–26078 (2015).
Fang, Y. & Flake, J. C. Electrochemical reduction of CO2 at functionalized Au electrodes. J. Am. Chem. Soc. 139, 3399–3405 (2017).
Luo, M. C. et al. Coordination polymer electrocatalysts enable efficient CO-to-acetate conversion. Adv. Mater. 35, 2209567 (2023).
Herrera, S. et al. Surface structure of 4-mercaptopyridine on Au (111): a new dense phase. Langmuir 33, 9565–9572 (2017).
Yang, H. B. et al. Atomically dispersed Ni (i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Nguyen, T. N. & Dinh, C.-T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 49, 7488–7504 (2020).
Feng, Y. et al. Te-doped Pd nanocrystal for electrochemical urea production by efficiently coupling carbon dioxide reduction with nitrite reduction. Nano Lett 20, 8282–8289 (2020).
Deng, S. et al. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 134, 4905–4917 (2012).
Bai, H. et al. Controllable CO adsorption determines ethylene and methane productions from CO2 electroreduction. Sci. Bull. 66, 62–68 (2021).
Bertheussen, E. et al. Acetaldehyde as an intermediate in the electroreduction of carbon monoxide to ethanol on oxide‐derived copper. Angew. Chem. 128, 1472–1476 (2016).
Gao, J. J. et al. Enabling direct H2O2 production in acidic media through rational design of transition metal single atom catalyst. Chem 3, 658–674 (2020).
Cai, W. Z. et al. Amorphous multimetal alloy oxygen evolving catalysts. ACS Mater. Lett. 2, 624–632 (2020).
He, M. et al. Selective enhancement of methane formation in electrochemical CO2 reduction enabled by a Raman-inactive oxygen-containing species on Cu. ACS Catal 12, 6036–6046 (2022).
Li, F. H., Wen, H. Q. & Tang, Q. Reaction mechanism and kinetics for carbon dioxide reduction on iron–nickel Bi-atom catalysts,. J. Mater. Chem. A 10, 13266–13277 (2022).
Acknowledgements
We acknowledge funding support from the City University of Kong Hong startup fund (9020003), ITF–RTH - Global STEM Professorship (9446006), the National Key Research and Development Program of China (No. 2022YFA1506200), CAS Project for Young Scientists in Basic Research (YSBR-022), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB36030200), the National Natural Science Foundation of China (Grant No. 22075195), the National Natural Science Foundation of China (No. 22208021, 21974103, 22102207, 2199152, and 21832004) and Photon Science Research center for Carbon Dioxide.
Author information
Authors and Affiliations
Contributions
J.D. and F.L. contributed equally. J.D., F.L., H.Y., T.Z. and B.L. conceived the project. B.L. supervised the project. J.D., X.R., Yu.L., Yi.L., Z.S., T.W., W.W., Y.W. and Y.C. performed the experimental study. T.Z., F.L. and Y.W. performed the theoretical study. J.D., F.L. H.Y., T.Z. and B.L. wrote the manuscript with support from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Cao-Thang Dinh and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, J., Li, F., Ren, X. et al. Molecular tuning boosts asymmetric C-C coupling for CO conversion to acetate. Nat Commun 15, 3641 (2024). https://doi.org/10.1038/s41467-024-47913-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-47913-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.