Abstract
This study investigated the potential associations between allergic diseases (asthma, allergic rhinitis, and atopic dermatitis) and the development of primary open-angle glaucoma. We utilized authorized data from the Korean National Health Information Database (KNHID), which provides comprehensive medical claims data and information from the National Health Screening Program. We compared the baseline characteristics of subjects with and without allergic diseases and calculated the incidence and risk of glaucoma development. Cox proportional hazard regression analysis was used to determine the risk of glaucoma development in subjects with allergic diseases. A total of 171,129 subjects aged 20–39 with or without allergic diseases who underwent a general health examination between 2009 and 2015 were included. Subjects with allergic diseases exhibited a higher incidence of glaucoma compared to the control group. The hazard ratio (HR) of glaucoma onset was 1.49 and 1.39 in subjects with at least one allergic disease before and after adjusting for potential confounding factors, respectively. Among allergic diseases, atopic dermatitis showed the highest risk for glaucoma development (aHR 1.73) after adjusting for confounders. Allergic rhinitis showed an increased risk for incident glaucoma after adjustment (aHR 1.38). Asthma showed the lowest but still increased risk for glaucoma (aHR 1.22). The associations were consistent in all subgroup analyses stratified by sex, smoking, drinking, exercise, diabetes, hypertension, dyslipidemia, or history of steroid. In conclusion, allergic diseases are associated with increased risk of glaucoma development. Among allergic diseases, atopic dermatitis showed the highest risk for glaucoma development followed by allergic rhinitis and asthma.
Similar content being viewed by others
Introduction
Allergic diseases are chronic inflammatory disorders triggered by allergens, resulting in immunological responses1. They pose a growing health and economic burden, with their prevalence rapidly increasing worldwide2. Allergic inflammation, induced by allergen exposure, can lead to various diseases, including asthma, allergic rhinitis (AR), anaphylaxis, urticaria, and atopic dermatitis (AD)3. Prolonged or repeated exposure to allergens can cause chronic inflammation not only at the exposed site but also throughout the body, indicating chronic systemic inflammation4.
Chronic systemic inflammation plays a critical role in neurodegenerative diseases5,6. Allergic inflammation can induce neuroinflammation by activating glial cells in the central and peripheral nervous systems7. Consequently, numerous studies have explored the links between allergic diseases and an increased risk of dementia, particularly Alzheimer’s disease, which shares pathophysiological characteristics with glaucoma in respect of cell death mechanisms, cognitive decline, depression, and autism spectrum disorders8,9,10,11.
Glaucoma, the leading cause of irreversible blindness worldwide, is projected to increase from 76.5 million in 2020 to 111.8 million in 204012,13. Although the prevalence of glaucoma increases with age, the prevalence of primary open-angle glaucoma in those aged 19–29 and 30–39 has been reported to be 1.2% and 2.4%, respectively, in a Nationwide study using the Korea National Health and Nutrition Examination Survey14. Identifying risk factors for early detection and treatment is important, especially in these young adults. Glaucoma is a progressive neurodegenerative disease, and neuroinflammation has emerged as an increasingly important risk factor for the development and progression of glaucoma15,16. In this regard, chronic systemic inflammation in allergic diseases may be associated with glaucoma pathogenesis. However, only a few studies have examined the associations between allergic diseases and glaucoma, reporting conflicting results4,7,17. Therefore, the purpose of this study was to evaluate the potential associations between various allergic diseases, namely asthma, AR, and AD, and the risk of glaucoma development in a large, Nationwide, longitudinal cohort database.
Method
Data source
This Nationwide population-based cohort study was approved by the Institutional Review Board of the Yeouido St. Mary’s Hospital, the Catholic University of Korea (SC22ZISE0064). The review board waived the requirement for informed consent because the data were publicly available and anonymous. This research adhered to the tenets of the Declaration of Helsinki. We used the Korean National Health Information Database (KNHID) provided by the Korean National Health Insurance Service (KNHIS). In Korea, all residents are required to be enrolled in the KNHIS. The KNHID includes the following health-related information: (i) demographics including anonymized code for each individual, age, gender, socioeconomic variables, household income, etc., (ii) comprehensive medical data including medical claims data based on diagnostic codes by the International Classification of Diseases 10th revision (ICD-10), admission and ambulatory care, treatment procedures, and prescription records, and (iii) data from the National Health Screening Program (NHSP). The NHSP is conducted by the KNHIS in all enrollees over the age of 20 biannually18. It includes basic health examination results such as anthropometric data, visual acuity, pure-tone audiometric testing, blood pressure, basic laboratory exams including fasting glucose and total cholesterol, and a standardized self-questionnaire regarding health-related lifestyle factors (smoking habits, alcohol consumption, and regular physical exercise).
Study population
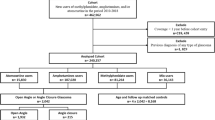
We included a total of 587,319 subjects who had undergone a general health examination at least once between 2009 and 2015. The date of the NHSP was regarded as the index date. Only those aged 20–39 were included in the study (n = 179,501). Those with missing data (n = 4552) and those with previously diagnosed glaucoma (n = 3820) were excluded. Previously diagnosed glaucoma was defined as diagnosis of glaucoma between January 1, 2002 and index date. In total, 171,129 subjects were included in the study and were followed using their medical records until December 31, 2018.
Allergic diseases were defined as at least 3 visits to the hospital with ICD-10 diagnostic code for AD (L20), AR (J301-304), or asthma (J45-46) as previously defined19 within one year prior to the index date. History of steroid was defined as any type of topical, inhaled, or oral steroid within 1 year of index date. Primary end point was development of primary open angle glaucoma, which was defined as at least 3 visits for glaucoma (H401) as previously defined20,21,22. The first diagnosis date was regarded as the occurrence date.
Health examinations were performed in hospitals certified by the KNHIS. If a subject underwent more than one health examination, only the first health examination was included for analysis. Anthropometric measurements were taken with the subjects wearing light clothing. Body mass index was calculated as the weight (kg) divided by the square of the height (m). Blood samples for serum glucose and lipid profiles including total cholesterol, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol were collected after an overnight fasting. Blood pressure was measured in a sitting position after a 5-min rest.
Comorbidities including diabetes (E11–E14), hypertension (I10, I11, I12, I13, and I15), and hypercholesterolemia (E78) were also defined based on ICD-10 codes and prescription history19,22. Smoking status was classified into non-smoker, ex-smoker, or current smoker, and alcohol consumption was classified into non-drinker (no alcoholic drinks within the past year), mild drinker (< 30 g of alcohol per day), or heavy drinker (≥ 30 g of alcohol per day). Subjects’ socioeconomic status was dichotomized into upper 80% and lower 20% based on household income, and area of residency was classified into urban or rural.
Statistical analysis
The baseline characteristics of the study subjects were compared using the student’s t-test for continuous variables and X2 test for categorical variables. The incidence rate of glaucoma was calculated by dividing the number of events by 1,000 person-years. Cox proportional hazard regression analysis was used to calculate the risk of glaucoma development according to the state of allergic diseases. Hazard ratio (HR) and 95% confidence interval (CI) was calculated before and after adjusting for potential confounding factors. Fully adjusted model included age, sex, income, hypertension, dyslipidemia, smoking status, alcohol consumption, regular exercise, body mass index (BMI), and history of steroid. In addition, we also calculated the risk of glaucoma development with at least one year lag after the index date to establish a temporal relationship. Subgroup analysis was performed after stratification according to sex, smoking status, alcohol consumption, regular exercise, comorbidities including diabetes, hypertension, dyslipidemia, and use of steroid. Interaction terms of allergic diseases with each stratification category were added to the Cox model to test the significance of the subgroup effects, and p values for interaction was calculated. Kaplan–Meier curve for incidence probability of glaucoma was generated.
Results
Baseline characteristics of the study population
A total of 171,129 subjects were included in the study. Table 1 shows the baseline characteristics of the study subjects. Subjects with at least one allergic disease (n = 23,758) were more likely female, in the lowest income quintile, non-smoker, non-drinker, older, lower BMI, lower glucose level, lower systolic and diastolic blood pressure, and better lipid profiles (lower total cholesterol, higher HDL, and lower LDL). Subjects with allergic disease were less likely to have comorbidities including hypertension and dyslipidemia, but not diabetes. Of note, subjects with at least one allergic disease were more likely to have received steroid treatment (topical, inhaled, or oral steroid) within 1 year of index date.
Incidence and risk of glaucoma among patients with allergic diseases
Table 2 shows the incidence and risk of glaucoma onset according to allergic diseases. The incidence of glaucoma was 4.50 and 7.08 per 1000 person-years in the control group and allergic disease group, respectively. Among allergic diseases, the incidence of glaucoma was 4.83 and 9.35 per 1000 person-years in the control group and AD group, respectively; 4.53 and 7.07 per 1000 person-years in the control group and AR group, respectively; and 4.82 and 7.07 per 1000 person-years in the control group and asthma group, respectively. The risk of glaucoma onset was 1.49 (95% CI 1.40–1.58) in subjects with at least one allergic disease before adjusting for potential confounding factors. After adjustment, the adjusted HR was 1.39 (95% CI 1.30–1.48). Among allergic diseases, AD showed the highest risk for glaucoma development before (HR 1.97, 95% CI 1.52–2.56) and after (aHR 1.73, 95% CI 1.34–2.24) adjustment. Allergic rhinitis showed increased risk for incident glaucoma before (HR 1.47, 95% CI 1.39–1.57) and after (aHR 1.38, 95% CI 1.29–1.47) adjusting for confounders. Among allergic diseases, asthma showed the lowest, but still increased risk for glaucoma before (HR 1.37, 95% CI 1.14–1.63) and after (aHR 1.22, 95% CI 1.02–1.46) adjustment. In addition, Supplementary Table 1 shows the risk of glaucoma including only those with at least one year lag between the index date and glaucoma, which shows similar results.
In subgroup analyses, adjusted HRs of study outcomes were compared in each subgroup stratified by sex, smoking, drinking, exercise, diabetes, hypertension, dyslipidemia, or history of steroid (Table 3). Overall, the association between allergic diseases and glaucoma onset was consistent in all subgroup analyses. In subjects with AD, those with dyslipidemia showed significantly greater risk of incident glaucoma (P for interaction = 0.01) compared to those without. In subjects with AR, those who are current smokers showed significantly greater risk of glaucoma development (P for interaction = 0.03) than those who are non-current smokers. In addition, those with history of steroid use showed significantly less prominent association with glaucoma onset compared to those without (P for interaction = 0.03). In subjects with asthma, the association between asthma and glaucoma was consistent in all subgroups. Figure 1 shows the cumulative glaucoma incidence according to allergic diseases.
Discussion
In this Nationwide longitudinal cohort study including young adults, we found significantly increased risk of glaucoma development in allergic diseases in general and in each of the three most common allergic diseases per se. Among allergic diseases, AD showed the greatest risk of subsequent glaucoma onset, followed by AR and asthma. Of note, the association was significant before and after adjusting for potential confounding factors including history of steroid use. The association between allergic diseases and subsequent glaucoma onset was robustly significant in all subgroups stratified by gender, lifestyle factors including smoking, drinking, and exercise, and systemic diseases including diabetes, hypertension, dyslipidemia, and history of steroid use.
There are few previous studies investigating the association between allergic diseases and glaucoma, with conflicting results. While glaucoma was not associated with allergic diseases in a Nationwide cross-sectional study using the Korean National Health and Nutrition Examination Survey4, it was associated with increased risk of AR using a population-based data in Taiwan17. In this study, Chung et al.17 hypothesized that the relationship between AR and glaucoma was mediated by autonomic dysfunction, as patients with AR were shown to have poor sympathetic modulation23, and nitric oxide, as high levels of nitric oxide were reported in patients with allergies24. In another study, Tseng et al.7 reported that glaucoma was associated with higher odds of sensitization to cockroach and cat allergens using the National Health and Nutrition Examination Survey, indicating that chronic exposure to certain allergens may play a role in the development of glaucoma. However, these studies were cross-sectional, limited to a particular allergic disease, or limited to self-reported allergic diseases. We found allergic diseases, namely AD, AR, and asthma, were in general and independently associated with increased risk of glaucoma development in a large Nationwide longitudinal cohort.
Although the exact pathophysiological mechanisms between allergic diseases and glaucoma remain unknown, there are some plausible etiologies. Chronic systemic inflammation is known to be associated with neurodegeneration12. Persistent or repetitive exposure to allergens in allergic diseases lead to chronic inflammation not only at the site of exposure, but even at other sites throughout the body3. Chronic systemic inflammation has been shown to stimulate glial cells to express proinflammatory cytokines including TNFα, IL-1, IL-4, IL-6, and IL-108. Elevated levels of these proinflammatory cytokines near the optic nerve head could potentially lead to neuroinflammation and neurodegeneration of the optic nerve. In line with this potential mechanism, the risk of incident dementia and Alzheimer's disease was increased in patients with asthma, AR, and AD, with dose–effect relationship with the severity of allergic diseases in a previous study8. The authors also explained the potential links between allergic diseases and dementia were chronic systemic inflammation and immune alterations affecting the brain. In a previous study, patients with glaucoma showed higher serum levels of IL-4 and IL-6 and those with severe optic neuropathy showed even higher levels, also suggesting the role of abnormal immune environment in the glaucoma pathogenesis25.
Oxidative stress in allergic diseases may also act as a link. Reactive oxygen species and reactive nitrogen species are increased in allergic diseases26. In asthma, not only airway, but also circulating inflammatory cells can secrete superoxide and contribute to elevated oxidative stress in asthma26,27. In addition, peripheral blood monocytes are primed to generate toxic oxygen metabolites in patients with severe AD28. Oxidative stress plays an important role in the pathogenesis of glaucoma causing trabecular meshwork degeneration and death of retinal ganglion cells29,30. In addition, allergic diseases have been linked with endothelial dysfunction, and therefore has been associated with systemic vascular diseases including diabetes, hypertension, and cardiovascular diseases8. Systemic vascular endothelial dysfunction has been associated with glaucoma, especially normal tension glaucoma which is the most prevalent type of primary open angle glaucoma in Korea31,32.
We found that among allergic diseases, AD showed the greatest risk of subsequent development of glaucoma. Repetitive and continuous eye rubbing can elevate intraocular pressure and has been associated with glaucomatous optic neuropathy33,34 Pecora and associates reported a case of a patient with progressive “normal tension glaucoma” which seemed to have been caused by recurrent eye rubbing. Progressive deterioration stabilized after the patient stopped the habit34.
In addition, steroid can cause elevation in IOP resulting in steroid-induced glaucoma, which may confound the association between allergic diseases and glaucoma35. Although steroid induced glaucoma (H406) may not likely be included in the analyses since we included only those with primary open angle glaucoma (H401), however, we attempted to further account for this by controlling for steroid in our analyses, which revealed consistent results. Furthermore, AR subjects with history of steroid use showed less prominent association with incident glaucoma compared to those without. Although exact mechanism remains unknown, potential mechanism may involve reduced inflammation by steroid. A previous study also reported decreased incidence of glaucoma in children with asthma using inhaled steroid36. The authors hypothesized that inhaled steroid reduced peripheral blood T cell activation and Th2-type cytokine mRNA expression and acted as immunosuppressive and anti-proliferative agent37.
Our sub analyses showed that in subjects with AD, those with dyslipidemia showed greater risk of incident glaucoma than those without. A previous study also reported that AD in adolescence was associated with dyslipidemia (high LDL)38,39. Hyperlipidemia can induce and potentiate proinflammatory cytokines and a Th2 response to external antigens, which may be a link between the two diseases40,41. Also, in subjects with AR, current smokers showed greater risk of glaucoma than non-current smokers. Smoking is a well-established risk factor for both AR and glaucoma42,43,44,45. Smoking has been associated with worsening of AR symptoms and increased inflammatory biomarkers in AR, which may be the potential link40,41.
The present study has several strengths. This is the first study showing significant associations between the three allergic diseases and glaucoma risk in a large Nationwide longitudinal data. We were able to assess the temporal relationship between various allergic diseases and onset of glaucoma in various subgroups. In addition, we used physician-diagnosed rather than self-reported diseases for exposures, outcomes, and comorbidities, increasing the validity of the diagnosis. We were also able to adjust for comprehensive risk factors for glaucoma including lifestyle factors (smoking, drinking, and exercise) and systemic diseases (hypertension and dyslipidemia).
Limitations
This study is subject to the following limitations. First, more detailed clinical information such as subtype or severity of diseases, which could lead to further understanding of the possible link between the two diseases, could not be assessed. Additionally, the possibility of detection bias cannot be ruled out. Patients with allergic diseases may visit hospitals more often, resulting in increased possibility of detecting glaucoma. In addition, our results were robust in all subgroups stratified by gender, lifestyle factors including smoking, drinking, and exercise, and systemic diseases including diabetes, hypertension, dyslipidemia, and history of steroid use.
Conclusion
In conclusion, allergic diseases including AD, AR, and asthma were significantly associated with increased risk of glaucoma development in young adults. Our findings have clinical implications: because glaucoma is an irreversible progressive disease, screening for glaucoma in those with allergic diseases can be an effective strategy for early glaucoma diagnosis.
Data availability
The data that support the findings of this study are available from the Korea National Health Insurance Sharing Service (NHISS) Institutional Data Access Committee (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do).
References
Aldakheel, F. M. Allergic diseases: A comprehensive review on risk factors, immunological mechanisms, link with COVID-19, potential treatments, and role of allergen bioinformatics. Int. J. Environ. Res. Public Health 18, 1–10 (2021).
Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 7, 12 (2014).
Galli, S. J., Tsai, M. & Piliponsky, A. M. The development of allergic inflammation. Nature 454, 445–454 (2008).
Lee, Y. B. et al. Association between allergic diseases and ophthalmologic diseases, including cataracts and glaucoma, using the Korean National Health and Nutrition Examination Survey 2010–2012: A STROBE-compliant article. J. Dermatol. 45, 463–467 (2018).
Wang, J., Song, Y., Chen, Z. & Leng, S. X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid. Med. Cell Longev. 2018, 1972714 (2018).
Sankowski, R., Mader, S. & Valdes-Ferrer, S. I. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front. Cell Neurosci. 9, 28 (2015).
Tseng, V. L., Lee, G. Y., Shaikh, Y., Yu, F. & Coleman, A. L. The association between glaucoma and immunoglobulin E antibody response to indoor allergens. Am. J. Ophthalmol. 159, 986–993 (2015).
Joh, H. K. et al. Allergic diseases and risk of incident dementia and Alzheimer’s disease. Ann. Neurol. 93, 384–397 (2023).
Fujii, T., Yamasaki, R. & Kira, J. I. Novel neuropathic pain mechanisms associated with allergic inflammation. Front. Neurol. 10, 1337 (2019).
Kanaya, A., Yang, M., Emala, C. & Mikami, M. Chronic allergic lung inflammation negatively influences neurobehavioral outcomes in mice. J. Neuroinflamm. 19, 210 (2022).
Park, D. Y., Kim, M., Bae, Y., Jang, H. & Lim, D. H. Risk of dementia in newly diagnosed glaucoma: A Nationwide cohort study in Korea. Ophthalmology 130, 684–691 (2023).
Baudouin, C., Kolko, M., Melik-Parsadaniantz, S. & Messmer, E. M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 83, 100916 (2021).
Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121, 2081–2090 (2014).
Shim, S. H. et al. The prevalence of open-angle glaucoma by age in myopia: The Korea national health and nutrition examination survey. Curr. Eye Res. 42, 65–71 (2017).
Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 87, 100998 (2022).
Quaranta, L. et al. Glaucoma and neuroinflammation: An overview. Surv. Ophthalmol. 66, 693–713 (2021).
Chung, S. D., Lin, H. C. & Hung, S. H. Allergic rhinitis is associated with open-angle glaucoma: A population-based case-control study. Am. J. Rhinol. Allergy 28, e148-151 (2014).
Cheol Seong, S. et al. Data resource profile: The National health information database of the national health insurance service in South Korea. Int. J. Epidemiol. 46, 799–800 (2017).
Han, J. H. et al. The risk of psoriasis in patients with allergic diseases: A Nationwide population-based cohort study. Allergy Asthma Immunol. Res. 13, 638–645 (2021).
Jung, Y., Han, K., Ohn, K., Kim, D. R. & Moon, J. I. Association between diabetes status and subsequent onset of glaucoma in postmenopausal women. Sci. Rep. 11, 18272 (2021).
Jung, Y. et al. Risk of cancer in patients with glaucoma: A Nationwide population-based cohort study. Sci. Rep. 10, 8170 (2020).
Jung, Y., Han, K., Park, H. Y. L., Lee, S. H. & Park, C. K. Metabolic health, obesity, and the risk of developing open-angle glaucoma: Metabolically healthy obese patients versus metabolically unhealthy but normal weight patients. Diabetes Metab. J. 44, 414–425 (2020).
Lan, M. Y., Lee, G. S., Shiao, A. S., Ko, J. H. & Shu, C. H. Heart rate variability analysis in patients with allergic rhinitis. Sci. World J. 2013, 947385 (2013).
Hiramoto, K., Kobayashi, H., Orita, K., Sato, E. F. & Ishii, M. Inducible nitric oxide synthase plays important roles in allergic reactions of pollinosis in mice sensitized with pollen allergy. J. Clin. Biochem. Nutr. 52, 17–21 (2013).
Huang, P. et al. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J. Glaucoma 19, 324–330 (2010).
Bowler, R. P. & Crapo, J. D. Oxidative stress in allergic respiratory diseases. J. Allergy Clin. Immunol. 110, 349–356 (2002).
Vachier, I. et al. Enhancement of reactive oxygen species formation in stable and unstable asthmatic patients. Eur. Respir. J. 7, 1585–1592 (1994).
Polla, B. S., Ezekowitz, R. A. & Leung, D. Y. Monocytes from patients with atopic dermatitis are primed for superoxide production. J. Allergy Clin. Immunol. 89, 545–551 (1992).
Izzotti, A., Bagnis, A. & Sacca, S. C. The role of oxidative stress in glaucoma. Mutat. Res. 612, 105–114 (2006).
Moreno, M. C. et al. Retinal oxidative stress induced by high intraocular pressure. Free Radic. Biol. Med. 37, 803–812 (2004).
Buckley, C., Hadoke, P. W., Henry, E. & O’Brien, C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br. J. Ophthalmol. 86, 227–232 (2002).
Kim, C. S., Seong, G. J., Lee, N. H., Song, K. C., Namil Study Group, K. G. S. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 118, 1024–1030 (2011).
Savastano, A., Savastano, M. C., Carlomusto, L. & Savastano, S. Bilateral glaucomatous optic neuropathy caused by eye rubbing. Case Rep. Ophthalmol. 6, 279–283 (2015).
Pecora, L., Sibony, P. & Fourman, S. Eye-rubbing optic neuropathy. Am. J. Ophthalmol. 134, 460–461 (2002).
Roberti, G. et al. Steroid-induced glaucoma: Epidemiology, pathophysiology, and clinical management. Surv. Ophthalmol. 65, 458–472 (2020).
Chang, L. S. et al. Decreased incidence of glaucoma in children with asthma using inhaled corticosteroid: A cohort study. Oncotarget 8, 105463–105471 (2017).
Gemou-Engesaeth, V., Bush, A., Kay, A. B., Hamid, Q. & Corrigan, C. J. Inhaled glucocorticoid therapy of childhood asthma is associated with reduced peripheral blood T cell activation and “Th2-type” cytokine mRNA expression. Pediatrics 99, 695–703 (1997).
Silverberg, J. I. Adult-onset atopic dermatitis. J. Allergy Clin. Immunol. Pract. 7, 28–33 (2019).
Hirano, T. & Matsunaga, K. Late-onset asthma: Current perspectives. J. Asthma Allergy 11, 19–27 (2018).
Stokes, K. Y., Cooper, D., Tailor, A. & Granger, D. N. Hypercholesterolemia promotes inflammation and microvascular dysfunction: Role of nitric oxide and superoxide. Free Radic. Biol. Med. 33, 1026–1036 (2002).
Al-Shawwa, B., Al-Huniti, N., Titus, G. & Abu-Hasan, M. Hypercholesterolemia is a potential risk factor for asthma. J. Asthma 43, 231–233 (2006).
Lin, S. Y., Reh, D. D., Clipp, S., Irani, L. & Navas-Acien, A. Allergic rhinitis and secondhand tobacco smoke: A population-based study. Am. J. Rhinol. Allergy 25, e66-71 (2011).
Grillo, C. et al. Influence of cigarette smoking on allergic rhinitis: A comparative study on smokers and non-smokers. Acta Biomed. 90, 45–51 (2019).
Jain, V., Jain, M., Abdull, M. M. & Bastawrous, A. The association between cigarette smoking and primary open-angle glaucoma: A systematic review. Int. Ophthalmol. 37, 291–301 (2017).
Solberg, Y., Rosner, M. & Belkin, M. The association between cigarette smoking and ocular diseases. Surv. Ophthalmol. 42, 535–547 (1998).
Funding
This research was supported by grant of the Institute of Clinical Medicine Research in the Yeouido St. Mary’s Hospital, The Catholic University of Korea. The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Conception and design: KH, JHJ, and YJ.; data acquisition: YJ; data analysis and interpretation: KH, JHJ, and YJ; statistical analysis: KH and JHJ; drafting and finalizing the article: KO and YJ.; critical revision of the article for important intellectual content: KH, JHJ, and JIM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, K., Jung, JH., Jung, Y. et al. The risk of open angle glaucoma in young adults with allergic diseases: a Nationwide cohort study. Sci Rep 14, 10694 (2024). https://doi.org/10.1038/s41598-024-57619-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57619-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.