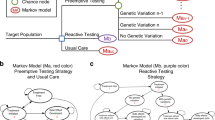
Abstract
We evaluated the cost-effectiveness of one-time pharmacogenomic testing for preventing adverse drug reactions (ADRs) over a patient’s lifetime. We developed a Markov-based Monte Carlo microsimulation model to represent the ADR events in the lifetime of each patient. The base-case considered a 40-year-old patient. We measured health outcomes in life years (LYs) and quality-adjusted LYs (QALYs) and estimated costs using 2013 US$. In the base-case, one-time genetic testing had an incremental cost-effectiveness ratio (ICER) of $43 165 (95% confidence interval (CI) is ($42 769,$43 561)) per additional LY and $53 680 per additional QALY (95% CI is ($53 182,$54 179)), hence under the base-case one-time genetic testing is cost-effective. The ICER values were most sensitive to the average probability of death due to ADR, reduction in ADR rate due to genetic testing, mean ADR rate and cost of genetic testing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP . Adverse drug events in hospitalized patientsExcess length of stay, extra costs, and attributable mortality. JAMA 1997; 277: 301–306.
De Smet PA, Denneboom W, Kramers C, Grol R . A composite screening tool for medication reviews of outpatients. Drugs Aging 2007; 24: 733–760.
Lazarou J, Pomeranz BH, Corey PN . Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–1205.
Drummond MF, O'Brien BJ, Stoddart GL, Torrance W . Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press: New York, NY, USA, 1998.
Gold MR, Siegel J, Russell L, Weinstein M . Cost-effectiveness in Health and Medicine: Report of the Panel on Cost-effectiveness in Health and Medicine. Oxford University Press: New York, NY, USA, 1996.
Heitjan D, Asch D, Ray R, Rukstalis M, Patterson F, Lerman C . Cost-effectiveness of pharmacogenetic testing to tailor smoking-cessation treatment. Pharmacogenomics J 2008; 8: 391–399.
Perlis RH, Patrick A, Smoller JW, Wang PS . When is pharmacogenetic testing for antidepressant response ready for the clinic? a cost-effectiveness analysis based on data from the STAR* D Study. Neuropsychopharmacology 2009; 34: 2227–2236.
Reese ES, Daniel Mullins C, Beitelshees AL, Onukwugha E . Cost-effectiveness of cytochrome p450 2c19 genotype screening for selection of antiplatelet therapy with clopidogrel or prasugrel. Pharmacotherapy 2012; 32: 323–332.
Alagoz O, Hsu H, Schaefer AJ, Roberts MS . Markov decision processes: a tool for sequential decision making under uncertainty. Med Decis Making 2009; 30: 474–483.
Bourgeois FT, Shannon MW, Valim C, Mandl KD . Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf 2010; 19: 901–910.
Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL . National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006; 296: 1858–1866.
Budnitz DS, Lovegrove MC, Shehab N, Richards CL . Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012.
Epstein RS, Moyer TP, Aubert RE, Kane DJ, Xia F, Verbrugge RR et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness Study). J Am Coll Cardiol 2010; 55: 2804–2812.
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. New England Journal of Medicine 2013; 369: 2283–2293.
Damani SB, Topol EJ . The case for routine genotyping in dual-antiplatelet therapy. J Am Coll Cardiol 2010; 56: 109–111.
Cause of Death 1999-2010 on CDC WONDER Online Database, released 2012. Data are from the Multiple Cause of Death Files, 1999-2010, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Available at http://wonder.cdc.gov/ucd-icd10.html. 2014.
Chyka PA . How many deaths occur annually from adverse drug reactions in the United States? Am J Med 2000; 109: 122–130.
Sarkar U, López A, Maselli JH, Gonzales R . Adverse drug events in US adult ambulatory medical care. Health Serv Res 2011; 46: 1517–1533.
Bond C, Raehl CL . Adverse drug reactions in United States hospitals. Pharmacotherapy 2006; 26: 601–608.
Neumann PJ . Why don’t Americans use cost-effectiveness analysis. Am J Manag Care 2004; 10: 308–312.
Chou WH, Yan F-X, de Leon J, Barnhill J, Rogers T, Cronin M et al. Extension of a pilot study: impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clinical Psychopharmacol 2000; 20: 246–251.
World Health Organization (2014) Table: Threshold values for intervention cost-effectiveness by Region. World Health Organization. Available at http://www.who.int/choice/costs/CER_levels/en/index.html. Accessed 7 November 2013.
Food and Drug Administration (2014). FDA Adverse Events Reporting System (FAERS) Patient Outcomes by Year 2014. Available at http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm070461.htm.
Rennie D, Luft HS . Pharmacoeconomic analyses: making them transparent, making them credible. JAMA 2000; 283: 2158–2160.
Wu C, Bell CM, Wodchis WP . Incidence and economic burden of adverse drug reactions among elderly patients in Ontario emergency departments. Drug Safety 2012; 35: 769–781.
Pfuntner A, Wier LM, Steiner C. Statistical brief #146 by Agency for Health Care Research and Quality: Costs for Hospital Stays in the United States, 2010. Agency for Health Care Policy and Research, Rockville, MD, USA, 2013. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf.
Shaw JW, Johnson JA, Coons SJ . US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005; 43: 203–220.
Centers for Disease Control Prevention (2013). CDC/NCHS National Hospital Discharge Survey, 2010. Number and rate of discharges from short-stay hospitals and days of care, with average length of stay and standard error, by selected first-listed diagnostic categories: United States, 2010. Available at http://www.cdc.gov/nchs/data/nhsr/nhsr029.pdf.
Agency for Healthcare Research and Quality (2011). Emergency Room Services-Mean and Median Expenses per Person With Expense and Distribution of Expenses by Source of Payment: United States. Medical Expenditure Panel Survey Household Component Data 2011. Available at: http://www.meps.ahrq.gov/mepsweb/surveycomp/household.jsp.
Acknowledgements
This research is funded by Renaissance Rx.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Alagoz, O., Durham, D. & Kasirajan, K. Cost-effectiveness of one-time genetic testing to minimize lifetime adverse drug reactions. Pharmacogenomics J 16, 129–136 (2016). https://doi.org/10.1038/tpj.2015.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.39
This article is cited by
-
Rates of genetic testing in patients prescribed drugs with pharmacogenomic information in FDA-approved labeling
The Pharmacogenomics Journal (2021)
-
Economic evaluation in psychiatric pharmacogenomics: a systematic review
The Pharmacogenomics Journal (2021)
-
Translating pharmacogenetics from research to routine clinical practice – a survey of the IGNITE Network
Translational Medicine Communications (2020)
-
Cost-effectiveness of precision medicine: a scoping review
International Journal of Public Health (2019)
-
On the readiness of physicians for pharmacogenomics testing: an empirical assessment
The Pharmacogenomics Journal (2018)