Abstract
Radiotherapy is one of the most common and effective therapies for cancer. Generally, patients are treated with X-rays produced by electron accelerators. Many years ago, researchers proposed that high-energy charged particles could be used for this purpose, owing to their physical and radiobiological advantages compared with X-rays. Particle therapy is an emerging technique in radiotherapy. Protons and carbon ions have been used for treating many different solid cancers, and several new centers with large accelerators are under construction. Debate continues on the cost:benefit ratio of this technique, that is, on whether the high costs of accelerators and beam delivery in particle therapy are justified by a clear clinical advantage. This Review considers the present clinical results in the field, and identifies and discusses the research questions that have resulted with this technique.
Key Points
-
Particle therapy is an emerging technique in radiotherapy, and several new centers are under construction all over the world
-
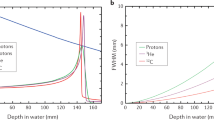
Protons are ideal for conformal treatment, and already have applications for pediatric tumors, where reduced late morbidity is expected owing to the reduced integral dose to normal tissue
-
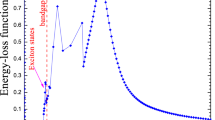
Heavy ions (carbon) provide not only physical, but also biological advantages compared with X-rays (such as high relative biological effectiveness and reduced oxygen enhancement ratio in the tumor region)
-
Clinical trials in Japan and Germany with carbon ions provided excellent results, especially for radiotherapy-resistant tumors, and suggest that hypofractionation is effective with particles
-
Spot scanning provides better dose profiles than passive beam modulation, but requires corrections for treating moving targets
-
Several research issues remain to be studied towards a wide application of heavy-ion therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lichter, A. S. & Lawrence, T. S. Recent advances in radiation oncology. N. Engl. J. Med. 332, 371–379 (1995).
Halperin, E. C. Particle therapy and treatment of cancer. Lancet Oncol. 7, 676–685 (2006).
Tobias, C. A. et al. Radiological physics characteristics of the extracted heavy ion beams of the Bevatron. Science 174, 1131–1134 (1971).
Cucinotta, F. A. & Durante, M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol. 7, 431–435 (2006).
Tobias, C. A. et al. Molecular and cellular radiobiology of heavy ions. Int. J. Radiat. Oncol. Biol. Phys. 8, 109–120 (1982).
Brahme, A. Recent advances in light ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 58, 603–616 (2004).
Nomiya, T. et al. Carbon ion radiation therapy for primary renal cell carcinoma: initial clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 72, 828–833 (2008).
Li, Q. et al. Heavy-ion conformal irradiation in shallow-seated tumor therapy terminal at HIRFL. Med. Biol. Eng. Comput. 45, 1037–1043 (2007).
Combs, S. E. Radiation therapy. Recent Results Cancer Res. 171, 125–140 (2009).
Particle Therapy Cooperative Group: an organization for those interested in proton, light ion and heavy charged particle radiotherapy [online], (2009).
Levin, W. P. et al. Proton beam therapy. Br. J. Cancer 93, 849–854 (2005).
Greco, C. & Wolden, S. Current status of radiotherapy with protons and light ion beams. Cancer 109, 1227–1238 (2007).
Yock, T. I. & Tarbell, N. J. Proton beam radiotherapy for treatment in pediatric brain tumors. Nat. Clin. Pract. Oncol. 1, 97–103 (2004).
Schulz-Ertner, D. & Tsujii, H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 25, 953–964 (2007).
Tsujii, H. et al. Clinical results of carbon ion radiotherapy at NIRS. J. Radiat. Res. 48, A1–A13 (2007).
Schulz-Ertner, D. et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int. J. Radiat. Oncol. Biol. Phys. 68, 449–457 (2007).
Tsujii, H. et al. Clinical advantages of carbon ion radiotherapy. N. J. Phys. 10, 075009 (2008).
Brada, M. et al. Proton therapy in clinical practice: current clinical evidence. J. Clin. Oncol. 25, 965–970 (2007).
Hegelich, B. M. et al. Laser acceleration of quasi-monoenergetic MeV ion beams. Nature 439, 441–444 (2006).
Kraft, G. & Kraft, S. D. Research needed for improving heavy-ion therapy. N. J. Phys. 11, 025001 (2009).
Parodi, K. et al. Comparison between in-beam and offline positron emission tomography imaging of proton and carbon ion therapeutic irradiation at synchrotron- and cyclotron-based facilities. Int. J. Radiat. Oncol. Biol. Phys. 71, 945–956 (2008).
Pedroni, E. et al. Experimental characterization and physical modelling of the dose distribution of scanned proton pencil beams. Phys. Med. Biol. 50, 541–561 (2005).
Saito, N. et al. Speed and accuracy of a beam tracking system for treatment of moving targets with scanned ion beams. Phys. Med. Biol. 54, 4849–4862 (2009).
Paganetti, H. et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 53, 407–421 (2002).
Gueulette, J. & Wambersie, A. Comparison of the methods of specifying carbon ion doses at NIRS and GSI. J. Radiat. Res. 48, A97–A102 (2007).
Matsufuji, N. et al. Specification of carbon ion dose at the National Institute of Radiological Sciences (NIRS). J. Radiat. Res. 48, A81–A86 (2007).
Elsässer, T. et al. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 71, 866–872 (2008).
Uzawa, A. et al. Comparison of biological effectiveness of carbon-ion beams in Japan and Germany. Int. J. Radiat. Oncol. Biol. Phys. 73, 1545–1551 (2009).
Kraft-Weyrather, W. et al. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int. J. Radiat. Biol. 75, 1357–1364 (1999).
Baumann, M. et al. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 8, 545–554 (2008).
Vazquez, A. et al. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat. Rev. Drug Discov. 7, 979–987 (2008).
Diehn, M. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458, 880–883 (2009).
Hamada, N. Recent insights into the biological action of heavy-ion radiation. J. Radiat. Res. 50, 1–9 (2009).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31 (1995).
Bernier, J. et al. Molecular therapy in head and neck oncology. Nat. Rev. Clin. Oncol. 6, 266–277 (2009).
Sonveaux, P. et al. Irradiaton-induced angiogenesis through the upregulation of the nitric oxide pathway: implications for tumor radiotherapy. Cancer Res. 63, 1012–1019 (2003).
Takahashi, Y. et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 63, 4253–4257 (2003).
Ogata, T. et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 65, 113–120 (2005).
Goetze, K. et al. The impact of conventional and heavy ion irradiation on tumor cell migration in vitro. Int. J. Radiat. Biol. 83, 889–896 (2007).
Akino, Y. et al. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 75, 475–481 (2009).
Rofstad, E. K. et al. Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-related upregulation of urokinase-type plasminogen activator receptor. Cancer Res. 64, 13–18 (2004).
Hall, E. J. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 65, 1–7 (2006).
Brenner, D. J. & Hall, E. J. Secondary neutrons in clinical proton radiotherapy: a charged issue. Radiother. Oncol. 86, 165–170 (2008).
St Clair, W. H. et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 58, 727–734 (2004).
Combs, S. E. et al. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer 115, 1348–1355 (2009).
Brenner, D. J. et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl Acad. Sci. USA 100, 13761–13766 (2003).
Durante, M. & Cucinotta, F. A. Heavy ion carcinogenesis and human space exploration. Nat. Rev. Cancer 8, 465–472 (2008).
Durante, M. et al. Karyotypes of human lymphocytes exposed to high-energy iron ions. Radiat. Res. 158, 581–590 (2002).
Durante, M. et al. X-rays vs. carbon-ion tumor therapy: cytogenetic damage in lymphocytes. Int. J. Radiat. Oncol. Biol. Phys. 47, 793–798 (2000).
Paganetti, H. The impact of protons on the incidence of second malignancies in radiotherapy by Eric J. Hall. Technol. Cancer Res. Treat. 6 (Suppl. 31–34), 661–662 (2007).
Chung, C. S. et al. Comparative analysis of secondary malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int. J. Radiat. Oncol. Biol. Phys. 72, S8 (2008).
Jäkel, O. et al. On the cost-effectiveness of carbon ion radiation therapy for skull base chordoma. Radiother. Oncol. 83, 133–138 (2007).
Jones, B. The case for particle therapy. Br. J. Radiol. 79, 24–31 (2006).
Konski, A. et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J. Clin. Oncol. 25, 3603–3608 (2007).
Suit, H. et al. Should positive phase III clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother. Oncol. 86, 148–153 (2008).
Brada, M. et al. Evidence for proton therapy. J. Clin. Oncol. 26, 2592–2593 (2008).
Jäkel, O. et al. The future of heavy ion therapy. Med. Phys. 35, 5653–5663 (2008).
Pijls-Johannesma, M. et al. Cost-effectiveness of particle therapy: current evidence and future needs. Radiother. Oncol. 89, 127–134 (2008).
Pijls-Johannesma, M. et al. Particle therapy in lung cancer: where do we stand? Cancer Treat. Rev. 34, 259–267 (2008).
Sugane, T. et al. Carbon ion radiotherapy for elderly patients 80 years and older with stage I non-small cell lung cancer. Lung Cancer 64, 45–50 (2009).
Miyamoto, T. et al. Curative treatment of stage I non-small cell lung cancer with carbon ion beams using a hypo-fractionated regimen. Int. J. Radiat. Oncol. Biol. Phys. 67, 750–758 (2007).
Miyamoto, T. et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J. Thorac. Oncol. 2, 916–926 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M. Durante declares no competing interests. J. Loeffler is a Consultant for Procure Inc.
Rights and permissions
About this article
Cite this article
Durante, M., Loeffler, J. Charged particles in radiation oncology. Nat Rev Clin Oncol 7, 37–43 (2010). https://doi.org/10.1038/nrclinonc.2009.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.183
This article is cited by
-
High efficacy of particle beam therapies against tumors under hypoxia and prediction of the early stage treatment effect using 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography
Annals of Nuclear Medicine (2024)
-
A spatial measure-valued model for radiation-induced DNA damage kinetics and repair under protracted irradiation condition
Journal of Mathematical Biology (2024)
-
Megaelectronvolt electron acceleration driven by terahertz surface waves
Nature Photonics (2023)
-
The ‘stealth-bomber’ paradigm for deciphering the tumour response to carbon-ion irradiation
British Journal of Cancer (2023)
-
Localized nuclear reaction breaks boron drug capsules loaded with immune adjuvants for cancer immunotherapy
Nature Communications (2023)