Featured
-
-
Article
| Open Access Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood
Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood
2-(2-Phenylethyl)chromones (PECs) contribute to the distinctive fragrance of agarwood. Here the authors identify a diarylpentanoid-producing polyketide synthase from Aquilaria sinensis and show how it catalyzes PEC formation.
- Xiao-Hui Wang
- , Bo-Wen Gao
- & She-Po Shi
-
Article
| Open Access Structure and function of a family of tick-derived complement inhibitors targeting properdin
Structure and function of a family of tick-derived complement inhibitors targeting properdin
Properdin is the only known positive regulator of the human complement system, stabilising the convertase C3 in the alternative pathway of complement activation. Here, the authors report the identification and characterisation of a species-specific properdin inhibitor CirpA, derived from tick saliva.
- Katharina Braunger
- , Jiyoon Ahn
- & Susan M. Lea
-
Article
| Open Access Molecular mechanism of agonism and inverse agonism in ghrelin receptor
Molecular mechanism of agonism and inverse agonism in ghrelin receptor
Ghrelin receptor regulates energy homeostasis through constitutive activity or by the ghrelin. Here the authors report two structures of ghrelin receptor bound to agonist and inverse agonist, providing insights into the mechanism of inverse agonism, which is of interest for specific ligand design.
- Jiao Qin
- , Ye Cai
- & Zhenhua Shao
-
Article
| Open Access Single particle cryo-EM structure of the outer hair cell motor protein prestin
Single particle cryo-EM structure of the outer hair cell motor protein prestin
Prestin, expressed in outer hair cell (OHC), belongs to the Slc26 transporter family and functions as a voltage-driven motor that drives OHC electromotility. Here, the authors report cryo-EM structure and characterization of gerbil prestin, with insights into its mechanism of action.
- Carmen Butan
- , Qiang Song
- & Joseph Santos-Sacchi
-
Article
| Open Access Structural basis of prokaryotic ubiquitin-like protein engagement and translocation by the mycobacterial Mpa-proteasome complex
Structural basis of prokaryotic ubiquitin-like protein engagement and translocation by the mycobacterial Mpa-proteasome complex
Pup is the bacterial analog of ubiquitin for targeting proteins to the proteasome. Here, the authors use cryoEM to visualize structures of the Mycobacterium tuberculosis proteasome translocating a Pup-tagged substrate.
- Mikhail Kavalchuk
- , Ahmad Jomaa
- & Eilika Weber-Ban
-
Article
| Open Access Structural insights in cell-type specific evolution of intra-host diversity by SARS-CoV-2
Structural insights in cell-type specific evolution of intra-host diversity by SARS-CoV-2
BriSΔ, a SARS-CoV-2 variant from clinical isolate hCoV/England/02/2020, comprises a deletion in a spike cleavage site. The structure and molecular dynamics of this spike provides mechanistic insights into how the deletion modulates virus infectivity.
- Kapil Gupta
- , Christine Toelzer
- & Imre Berger
-
Article
| Open Access Mapping inhibitory sites on the RNA polymerase of the 1918 pandemic influenza virus using nanobodies
Mapping inhibitory sites on the RNA polymerase of the 1918 pandemic influenza virus using nanobodies
Influenza viruses carry their own RNAdependent RNA-polymerase that is highly conserved and a promising anti-viral target. Combining functional and structural data, Keown et al. characterise the inhibitory effect of nanobodies on 1918 pandemic H1N1 influenza strain polymerase complex and identify sensitive sites interfering with polymerase activity in vitro.
- Jeremy R. Keown
- , Zihan Zhu
- & Jonathan M. Grimes
-
Article
| Open Access Structural basis of BAK activation in mitochondrial apoptosis initiation
Structural basis of BAK activation in mitochondrial apoptosis initiation
The authors show that the mechanism of BAK activation in mitochondrial apoptosis involves cooperation between direct activation by BH3-only protein BID and BAK autoactivation, providing a unifying basis for BAK triggering by BH3 ligands.
- Geetika Singh
- , Cristina D. Guibao
- & Tudor Moldoveanu
-
Article
| Open Access A small RNA that cooperatively senses two stacked metabolites in one pocket for gene control
A small RNA that cooperatively senses two stacked metabolites in one pocket for gene control
Riboswitches contain an aptamer domain that recognizes a metabolite and an expression platform that regulates gene expression. Here the authors report the crystal structure of a preQ1-sensing riboswitch from Carnobacterium antarcticus that shows two metabolites in a single binding pocket.
- Griffin M. Schroeder
- , Chapin E. Cavender
- & Joseph E. Wedekind
-
Article
| Open Access Neutron crystallography reveals mechanisms used by Pseudomonas aeruginosa for host-cell binding
Neutron crystallography reveals mechanisms used by Pseudomonas aeruginosa for host-cell binding
Pseudomonas aeruginosa employs lectins to bind to its host cells, and is known to be the major cause of lung infections. Lectin B (LecB) from Pseudomonas aeruginosa binds specifically to galactose and fucose and is important for pathogenicity, adhesion and biofilm formation. In this work, the neutron crystal structure (1.9 Å) of the deuterated LecB/Ca/fucose complex is reported. The structure, in combination with perdeuteration of the ligand and the receptor allowed the observation of hydrogen atoms, protonation states and hydrogen bonds involved in the interaction between pathogenic bacteria and host cells. Thus the study provides structural insights into the mechanism of high affinity binding of LecB to its targets.
- Lukas Gajdos
- , Matthew P. Blakeley
- & Anne Imberty
-
Article
| Open Access Design principles for site-selective hydroxylation by a Rieske oxygenase
Design principles for site-selective hydroxylation by a Rieske oxygenase
SxtT and GxtA are Rieske oxygenases that are involved in paralytic shellfish toxin biosynthesis and catalyze monohydroxylation reactions at different positions on the toxin scaffold. Here, the authors present crystal structures of SxtT and GxtA with the native substrates β-saxitoxinol and saxitoxin as well as a Xenon-pressurized structure of GxtA, which reveal a substrate access tunnel to the active site. Through structure-based mutagenesis studies the authors identify six residues in three different protein regions that determine the substrate specificity and site selectivity of SxtT and GxtA. These findings will aid the rational engineering of other Rieske oxygenases.
- Jianxin Liu
- , Jiayi Tian
- & Jennifer Bridwell-Rabb
-
Article
| Open Access Topoisomerase I (TOP1) dynamics: conformational transition from open to closed states
Topoisomerase I (TOP1) dynamics: conformational transition from open to closed states
Topoisomerase I (TOP1) relaxes both positive and negative supercoils by nicking DNA and after rotation of the broken DNA strand closes the nick. Here, the authors present the DNA free crystal structure of TOP1 from the hyperthermophilic archaeon Caldiarchaeum subterraneum in the open form and discuss the mechanism of how DNA enters the catalytic site of TOP1.
- Diane T. Takahashi
- , Danièle Gadelle
- & Claudine Mayer
-
Article
| Open Access Crystal structure and functional implication of bacterial STING
Crystal structure and functional implication of bacterial STING
The bacterial Cyclic-oligonucleotide-Based Anti-phage Signaling System (CBASS) contains a CD-NTase that synthesizes cyclic di- and tri-nucleotides, and bacterial STING proteins recognize c-di-GMP generated by CD-NTase during phage infection and signal the infected bacteria to commit suicide. Here, the authors provide insights into the molecular basis for c-di-GMP recognition of bacterial STING proteins by determining two STING protein crystal structures with bound c-di-GMP from Prevotella corporis and Myroides sp. ZB35.
- Tzu-Ping Ko
- , Yu-Chuan Wang
- & Yeh Chen
-
Article
| Open Access Cryo-EM structure of native human thyroglobulin
Cryo-EM structure of native human thyroglobulin
The iodinated thyroglobulin functions as iodine storage and carrier protein and a precursor for thyroid hormone (TH) biogenesis. Here, the authors report the structure of native, fully glycosylated human thyroglobulin, revealing the location of the hTg hormonogenic and glycosylation sites.
- Ricardo Adaixo
- , Eva M. Steiner
- & Nicholas M. I. Taylor
-
Article
| Open Access Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract
Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract
Triclosan (TCS), an antimicrobial agent commonly found in consumer products, has been reported to exacerbates colitis in animal models. Here, using in vitro and in vivo approaches, the authors show that gut bacterial enzymes can drive the metabolic activation and gut toxicity of TCS, highlighting an important role of intestinal microbial factors in the complex etiology of colitis.
- Jianan Zhang
- , Morgan E. Walker
- & Guodong Zhang
-
Article
| Open Access Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation
Dynamics of GLP-1R peptide agonist engagement are correlated with kinetics of G protein activation
The glucagon-like peptide-1 receptor (GLP-1R) can be targeted in the treatment of diabetes, obesity and other metabolic disorders. Here, the authors assess the molecular mechanisms of peptide agonists binding to GLP-1R and the responses elucidated by these ligands, including distinct kinetics of G protein activation.
- Giuseppe Deganutti
- , Yi-Lynn Liang
- & Denise Wootten
-
Article
| Open Access Harnessing protein folding neural networks for peptide–protein docking
Harnessing protein folding neural networks for peptide–protein docking
AlphaFold2 has originally been developed to provide highly accurate predictions of protein monomer structures. Here, the authors present a simple adaptation of AlphaFold2 that enables structural modeling of peptide–protein complexes, and explore the underlying mechanisms and limitations of this approach.
- Tomer Tsaban
- , Julia K. Varga
- & Ora Schueler-Furman
-
Article
| Open Access Hypocrates is a genetically encoded fluorescent biosensor for (pseudo)hypohalous acids and their derivatives
Hypocrates is a genetically encoded fluorescent biosensor for (pseudo)hypohalous acids and their derivatives
There are a lack of tools to study the dynamics of (pseudo)hypohalous acids in live cells. Here the authors report a genetically encoded fluorescent biosensor, Hypocrates, for (pseudo)hypohalous acids and their derivatives which they use in cells and in a zebrafish tail fin injury model.
- Alexander I. Kostyuk
- , Maria-Armineh Tossounian
- & Vsevolod V. Belousov
-
Article
| Open Access The catalytic activity of TCPTP is auto-regulated by its intrinsically disordered tail and activated by Integrin alpha-1
The catalytic activity of TCPTP is auto-regulated by its intrinsically disordered tail and activated by Integrin alpha-1
TCPTP is a non-receptor type protein tyrosine phosphatase involved in various signalling pathways. Here, the authors provide structural insights into TCPTP activation, showing that TCPTP is inhibited by its C-terminal tail, which can be displaced by the cytosolic tail of integrin-α1, leading to activation.
- Jai Prakash Singh
- , Yang Li
- & Tzu-Ching Meng
-
Article
| Open Access Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation
Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation
Tyrosine hydroxylase (TH) catalyzes the rate-limiting step in the synthesis of the catecholamine neurotransmitters and hormones dopamine (DA), adrenaline and noradrenaline. Here, the authors present the cryo-EM structures of full-length human TH in the apo form and bound with DA, as well as the structure of Ser40 phosphorylated TH, and discuss the inhibitory and stabilizing effects of DA on TH and its counteraction by Ser40-phosphorylation.
- María Teresa Bueno-Carrasco
- , Jorge Cuéllar
- & José M. Valpuesta
-
Article
| Open Access Structural assessment of HLA-A2-restricted SARS-CoV-2 spike epitopes recognized by public and private T-cell receptors
Structural assessment of HLA-A2-restricted SARS-CoV-2 spike epitopes recognized by public and private T-cell receptors
Structural immunology is critical in understanding the interplay between the immune response and the infective agent but such studies in T cells and SARS-CoV-2 lag behind those of antibodies and B-cell receptors. Here the authors assess recognition of SARS-CoV-2 spike epitopes and their natural variants by public and private T cell receptors.
- Daichao Wu
- , Alexander Kolesnikov
- & Roy A. Mariuzza
-
Article
| Open Access Cryo-EM demonstrates the in vitro proliferation of an ex vivo amyloid fibril morphology by seeding
Cryo-EM demonstrates the in vitro proliferation of an ex vivo amyloid fibril morphology by seeding
Here, the authors present the cryo-EM structure of in vitro amyloid fibrils from recombinant SAA1.1 protein that were formed by seeding with fibrils purified from systemic AA amyloidosis tissue. This in vitro fibril structure resembles the structure of the ex vivo fibrils but differs from unseeded in vitro fibrils. These findings show that fibril morphologies can be propagated in vitro by seeding.
- Thomas Heerde
- , Matthies Rennegarbe
- & Marcus Fändrich
-
Article
| Open Access A method to construct the dynamic landscape of a bio-membrane with experiment and simulation
A method to construct the dynamic landscape of a bio-membrane with experiment and simulation
The authors present a strategy to construct dynamic biomolecular landscapes. Here, they derive a quantitative description of the distribution timescales and amplitudes of reorientational motion of POPC membranes from the combination of NMR relaxation data and frame analysis of MD simulations.
- Albert A. Smith
- , Alexander Vogel
- & Daniel Huster
-
Article
| Open Access Conformational dynamics of the Beta and Kappa SARS-CoV-2 spike proteins and their complexes with ACE2 receptor revealed by cryo-EM
Conformational dynamics of the Beta and Kappa SARS-CoV-2 spike proteins and their complexes with ACE2 receptor revealed by cryo-EM
Here, the authors provide insights into the conformational dynamics of the Beta and Kappa SARS-CoV-2 spike (S) proteins by determining their cryo-EM structures, which revealed a distribution shift towards the open state for both variants compared to the wild-type S protein. They also present the structures of the Kappa and Beta S-ACE2 complexes, where a population shift towards the three receptor-binding domain up conformation was observed. In combination with biochemical data these structures show how the S protein variants efficiently recognize and bind to ACE2.
- Yifan Wang
- , Cong Xu
- & Yao Cong
-
Article
| Open Access Structure-guided bifunctional molecules hit a DEUBAD-lacking hRpn13 species upregulated in multiple myeloma
Structure-guided bifunctional molecules hit a DEUBAD-lacking hRpn13 species upregulated in multiple myeloma
Rpn13 is a substrate receptor of the 26S proteasome and an anti-cancer drug target. Here, the authors identify and characterize XL5, a lead compound that binds to the N-terminal Pru domain of human Rpn13 (hRpn13), solve the NMR structure of XL5-ligated hRpn13 Pru and develop XL5-PROTACs that preferentially target an identified hRpn13 Pru fragment present in multiple myeloma cells.
- Xiuxiu Lu
- , Venkata R. Sabbasani
- & Kylie J. Walters
-
Article
| Open Access Nanoparticles and photochemistry for native-like transmembrane protein footprinting
Nanoparticles and photochemistry for native-like transmembrane protein footprinting
The intrinsic flexibility of membranes proteins still poses a challenge in determining their active structure. Here the authors describe the development of a method that combines chemical footprinting and mass spectrometry to assist in determining the structure of native membrane proteins and their dynamics.
- Jie Sun
- , Xiaoran Roger Liu
- & Michael L. Gross
-
Article
| Open Access Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP
Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP
EF-G drives ribosomal translocation along mRNA. Time-resolved cryo-EM captured translocation with EF-G•GTP—without inhibitors—revealing how EF-G uses ribosome fluctuations to drive translocation and GTP hydrolysis to leave at the right moment.
- Christine E. Carbone
- , Anna B. Loveland
- & Andrei A. Korostelev
-
Article
| Open Access How to build a ribosome from RNA fragments in Chlamydomonas mitochondria
How to build a ribosome from RNA fragments in Chlamydomonas mitochondria
Mitoribosomes are remarkably diverse in their structures and compositions. Here the authors combine biochemistry, genetics, single particle cryo-electron microscopy and in situ cryo-electron tomography to reveal the mitochondrial ribosome of Chlamydomonas reinhardtii as an extreme example of evolution and species-specific adaptation.
- Florent Waltz
- , Thalia Salinas-Giegé
- & Yaser Hashem
-
Article
| Open Access Structural and molecular basis for Cardiovirus 2A protein as a viral gene expression switch
Structural and molecular basis for Cardiovirus 2A protein as a viral gene expression switch
Many RNA viruses employ programmed –1 ribosomal frameshifting (PRF) to expand their coding capacity and optimize production of viral proteins. Here, the authors report structural and biophysical analysis of protein 2A from a cardiovirus, with insights into the mechanism of its PRF-stimulatory function.
- Chris H. Hill
- , Lukas Pekarek
- & Ian Brierley
-
Article
| Open Access Hedgehog-Interacting Protein is a multimodal antagonist of Hedgehog signalling
Hedgehog-Interacting Protein is a multimodal antagonist of Hedgehog signalling
Hedgehog-Interacting Protein (HHIP) is the only reported secreted inhibitor of Sonic Hedgehog (SHH) signalling. Here, the authors report structures of the HHIP N- and C-terminal domains, both in complexes with glycosaminoglycans, providing insights into the molecular basis for SHH sequestration and inhibition.
- Samuel C. Griffiths
- , Rebekka A. Schwab
- & Christian Siebold
-
Article
| Open Access Biphasic activation of β-arrestin 1 upon interaction with a GPCR revealed by methyl-TROSY NMR
Biphasic activation of β-arrestin 1 upon interaction with a GPCR revealed by methyl-TROSY NMR
β-arrestins commonly bind to two distinct elements in GPCRs: the phosphorylated carboxyl terminal tail (C tail) and the cytoplasmic face of the transmembrane region (TM core). Here, the authors use methyl-TROSY NMR measurements to characterise the interactions between β-arrestin 1 (βarr1) and a GPCR and observe that C tail-mediated interaction with a GPCR alone induces the partial activation of βarr1, whereas the TM core- and C tail-mediated interactions together stabilize the activated conformation of βarr1.
- Yutaro Shiraishi
- , Yutaka Kofuku
- & Ichio Shimada
-
Article
| Open Access Small molecule modulation of the Drosophila Slo channel elucidated by cryo-EM
Small molecule modulation of the Drosophila Slo channel elucidated by cryo-EM
Slowpoke (Slo) channels are voltage-gated potassium channels that are activated by high intracellular Ca2+ concentrations, and they are targets for insecticides and antiparasitic drugs. Here, the authors present the cryo-EM structures of the Drosophila melanogaster Slo channel in the Ca2+-bound and Ca2+-free conformations, as well as in complex with the fungal neurotoxin verruculogen and the anthelmintic drug emodepside and discuss the mechanisms by which they affect the activity of Slo.
- Tobias Raisch
- , Andreas Brockmann
- & Stefan Raunser
-
Article
| Open Access Structural basis for transthyretin amyloid formation in vitreous body of the eye
Structural basis for transthyretin amyloid formation in vitreous body of the eye
Systemic ATTR amyloidosis causes the abnormal accumulation of ATTR fibrils formed from the human plasma protein transthyretin (TTR) in multiple organs including the eye. Here, the authors present a 3.2 Å cryo-EM structure of an ATTR fibril isolated from the vitreous body of an ATTR patient’s eye and discuss the mechanism for the structural conversion of TTR into a fibrillar form.
- Irina Iakovleva
- , Michael Hall
- & A. Elisabeth Sauer-Eriksson
-
Article
| Open Access Sliding of HIV-1 reverse transcriptase over DNA creates a transient P pocket – targeting P-pocket by fragment screening
Sliding of HIV-1 reverse transcriptase over DNA creates a transient P pocket – targeting P-pocket by fragment screening
Here the authors observe a transient P-pocket created when HIV reverse transcriptase slides over DNA substrate, identify fragments targeting this pocket, and develop a cryo-EM platform for lead optimization.
- Abhimanyu K. Singh
- , Sergio E. Martinez
- & Kalyan Das
-
Article
| Open Access Plasticity within the barrel domain of BamA mediates a hybrid-barrel mechanism by BAM
Plasticity within the barrel domain of BamA mediates a hybrid-barrel mechanism by BAM
The β-barrel assembly machinery (BAM) assists the folding and membrane insertion of bacterial outer membrane proteins. Here, the authors report structural characterization of BAM in lipid environment and in complex with the client protein EspP integrated into the barrel of BamA, providing insight into BAM mechanism of function.
- Runrun Wu
- , Jeremy W. Bakelar
- & Nicholas Noinaj
-
Article
| Open Access Molecular basis for redox control by the human cystine/glutamate antiporter system xc−
Molecular basis for redox control by the human cystine/glutamate antiporter system xc−
System xc- is a cystine transporter that is expressed in the plasma membrane and imports cystine in exchange for intracellular glutamate. Here, the authors present the cryo-EM structure of human system xc- both in the apo form and the glutamate bound state, and further supported by molecular dynamics and cell-based assays they discuss its cystine transport mechanism.
- Joanne L. Parker
- , Justin C. Deme
- & Simon Newstead
-
Article
| Open Access eIF2B-capturing viral protein NSs suppresses the integrated stress response
eIF2B-capturing viral protein NSs suppresses the integrated stress response
Here the authors show that a viral protein interferes with the binding of phosphorylated eIF2 to eIF2B, thereby suppressing the host integrated stress response (ISR). This suppression of the ISR abrogates translational changes of the host and ameliorates neurite degradation under stress.
- Kazuhiro Kashiwagi
- , Yuichi Shichino
- & Takuhiro Ito
-
Article
| Open Access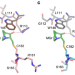 Structural basis of reactivation of oncogenic p53 mutants by a small molecule: methylene quinuclidinone (MQ)
Structural basis of reactivation of oncogenic p53 mutants by a small molecule: methylene quinuclidinone (MQ)
The tumor suppressor p53 is mutated in more than half of human cancers and the compound methylene quinuclidinone (MQ) was shown to reactivate p53 mutants by binding covalently to cysteine residues. Here, the authors present crystal structures of wild-type and cancer related p53 mutant core domains bound to MQ alone and in complex with their DNA response elements and observe that MQ is bound to several cysteine residues located at the surface of the core domain.
- Oksana Degtjarik
- , Dmitrij Golovenko
- & Zippora Shakked
-
Article
| Open Access DeepRank: a deep learning framework for data mining 3D protein-protein interfaces
DeepRank: a deep learning framework for data mining 3D protein-protein interfaces
The authors present DeepRank, a deep learning framework for the data mining of large sets of 3D protein-protein interfaces (PPI). They use DeepRank to address two challenges in structural biology: distinguishing biological versus crystallographic PPIs in crystal structures, and secondly the ranking of docking models.
- Nicolas Renaud
- , Cunliang Geng
- & Li C. Xue
-
Article
| Open Access Mechanism of phosphate sensing and signaling revealed by rice SPX1-PHR2 complex structure
Mechanism of phosphate sensing and signaling revealed by rice SPX1-PHR2 complex structure
SPX proteins sense phosphate levels in plant cells by binding to inositol polyphosphates (InsP) and suppressing the activity of PHR transcription factors. Here the authors show that when bound to InsP6, the rice SPX1 protein inhibits the activity of PHR2 by attenuating both its dimerization and DNA binding activity.
- Jia Zhou
- , Qinli Hu
- & Weiman Xing
-
Article
| Open Access Regulation of the EphA2 receptor intracellular region by phosphomimetic negative charges in the kinase-SAM linker
Regulation of the EphA2 receptor intracellular region by phosphomimetic negative charges in the kinase-SAM linker
Eph receptor tyrosine kinases and their ephrin ligands mediate cell-cell communication. Here, the authors assess the structure and dynamics of the EphA2 intracellular region and uncover complex effects of phosphorylation within the linker region between EphA2 kinase and SAM domains.
- Bernhard C. Lechtenberg
- , Marina P. Gehring
- & Elena B. Pasquale
-
Article
| Open Access Discovery of an exosite on the SOCS2-SH2 domain that enhances SH2 binding to phosphorylated ligands
Discovery of an exosite on the SOCS2-SH2 domain that enhances SH2 binding to phosphorylated ligands
SOCS2 is a key regulator of growth hormone and cytokine signaling, which recognizes phosphotyrosine (pTyr)-modified targets via a central SH2 domain. Here, the authors discover and characterize an exosite on this SH2 domain that can bind a non-phosphorylated peptide to enhance SOCS2:pTyr affinity.
- Edmond M. Linossi
- , Kunlun Li
- & Sandra E. Nicholson
-
Article
| Open Access Catalytic flexibility of rice glycosyltransferase OsUGT91C1 for the production of palatable steviol glycosides
Catalytic flexibility of rice glycosyltransferase OsUGT91C1 for the production of palatable steviol glycosides
Steviol glycosides from the plant Stevia rebaudiana are already used as lowcalorie sweeteners, but the most abundant naturally occurring compounds have a bitter aftertaste. Here, the authors characterize and engineer rice glycosyltransferase OsUGT91C1 to facilitate the large-scale production of naturally rare but palatable glycosides Reb D and Reb M
- Jinzhu Zhang
- , Minghai Tang
- & Wei Cheng
-
Article
| Open Access Conformational changes in Lassa virus L protein associated with promoter binding and RNA synthesis activity
Conformational changes in Lassa virus L protein associated with promoter binding and RNA synthesis activity
The L protein of segmented, negative strand RNA viruses contains the RNA-dependent RNA polymerase essential for virus amplification. Here, the authors report cryoEM structures of the Lassa virus L protein in active, RNA-bound states, and provide mechanistic insights.
- Tomas Kouba
- , Dominik Vogel
- & Stephen Cusack
-
Article
| Open Access Kinetic and structural mechanism for DNA unwinding by a non-hexameric helicase
Kinetic and structural mechanism for DNA unwinding by a non-hexameric helicase
UvrD is a model helicase from the non-hexameric Superfamily 1. Here, the authors use optical tweezers to measure directly the stepwise translocation of UvrD along a DNA hairpin, and propose a mechanism in which UvrD moves one base pair at a time, but sequesters the nascent single strands, releasing them after a variable number of ATP hydrolysis cycles.
- Sean P. Carney
- , Wen Ma
- & Yann R. Chemla
-
Article
| Open Access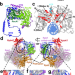 Mechanism of Rad26-assisted rescue of stalled RNA polymerase II in transcription-coupled repair
Mechanism of Rad26-assisted rescue of stalled RNA polymerase II in transcription-coupled repair
Here the authors provide models of RNA polymerase II bound to the yeast CSB ortholog Rad26 in different nucleotide states; explain how Rad26 domain motions help the polymerase progress past DNA lesions; and interpret the effects of CSB-associated disease mutations.
- Chunli Yan
- , Thomas Dodd
- & Ivaylo Ivanov
-
Article
| Open Access Mounting, structure and autocleavage of a type VI secretion-associated Rhs polymorphic toxin
Mounting, structure and autocleavage of a type VI secretion-associated Rhs polymorphic toxin
Rearrangement hot spots (Rhs) proteins are bacterial polymorphic toxin systems. Here, the authors show that Rhs1 forms a complex with the Type VI secretion system (T6SS) spike protein VgrG and the EagR chaperone. They also present the cryo-EM structure of the Rhs1-EagR complex and propose a model for Rhs loading and delivery by the T6SS.
- Dukas Jurėnas
- , Leonardo Talachia Rosa
- & Eric Cascales
-
Article
| Open Access Structural basis for the E3 ligase activity enhancement of yeast Nse2 by SUMO-interacting motifs
Structural basis for the E3 ligase activity enhancement of yeast Nse2 by SUMO-interacting motifs
Nse2 is a SUMO E3 ligase component of the Smc5/6 multisubunit complex involved in the DNA repair and chromosome integrity. Here, the structure of the Nse2 in complex with an E2-SUMO thioester mimetic reveals the combined action of two SIM motifs during the E3- dependent conjugation reaction.
- Nathalia Varejão
- , Jara Lascorz
- & David Reverter
-
Article
| Open Access Crystal structures of phosphatidyl serine synthase PSS reveal the catalytic mechanism of CDP-DAG alcohol O-phosphatidyl transferases
Crystal structures of phosphatidyl serine synthase PSS reveal the catalytic mechanism of CDP-DAG alcohol O-phosphatidyl transferases
CDP-diacylglycerol (CDP-DAG) alcohol O-phosphatidyl transferases (CDP-APs) are conserved in archaea, bacteria, and eukaryotes and catalyze the de novo synthesis of phospho-lipids from the precursor CDP-DAG and an alcohol. Here, the authors present the crystal structures of the Methanocaldococcus jannaschii phosphatidyl serine synthase (MjPSS) in four different states and suggest a model for its catalytic mechanism.
- Martin Centola
- , Katharina van Pee
- & Özkan Yildiz

 Crystal structure of the α1B-adrenergic receptor reveals molecular determinants of selective ligand recognition
Crystal structure of the α1B-adrenergic receptor reveals molecular determinants of selective ligand recognition