Featured
-
-
Article
| Open Access Phenylalanine-tRNA aminoacylation is compromised by ALS/FTD-associated C9orf72 C4G2 repeat RNA
Phenylalanine-tRNA aminoacylation is compromised by ALS/FTD-associated C9orf72 C4G2 repeat RNA
GGGGCC repeat expansion in the C9orf72 gene causes amyotrophic lateral sclerosis and frontotemporal dementia. Here the authors show that CCCCGG antisense repeat RNA binds and inhibits phenylalanine-tRNA synthetase resulting in decreased levels of tRNAphe and phenylalanine rich proteins.
- Mirjana Malnar Črnigoj
- , Urša Čerček
- & Boris Rogelj
-
Article
| Open Access At-home wearables and machine learning sensitively capture disease progression in amyotrophic lateral sclerosis
At-home wearables and machine learning sensitively capture disease progression in amyotrophic lateral sclerosis
“In ALS clinical trials, efficacy is often assessed via subjective patient or clinician reports. The authors introduce a machine-learned severity score based on wearable sensor data from daily activities, which is reliable, sensitive, and could reduce clinical trial sizes.”
- Anoopum S. Gupta
- , Siddharth Patel
- & Fernando Vieira
-
Article
| Open Access Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis
Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis
Neuroinflammation and autophagy are two pillars of ALS pathogenesis targeted by rapamycin. Here, in a randomized, double-blind, phase 2 clinical trial, the authors find rapamycin to be safe and well tolerated in ALS patients, supporting further studies.
- Jessica Mandrioli
- , Roberto D’Amico
- & Andrea Cossarizza
-
Article
| Open Access Integrated transcriptome landscape of ALS identifies genome instability linked to TDP-43 pathology
Integrated transcriptome landscape of ALS identifies genome instability linked to TDP-43 pathology
The causes of ALS remain unclear with many proposed pathomechanisms. Here, the authors integrate iPSC-derived motor neuron and post-mortem datasets and identify a heightened DNA damage response accompanied by accumulation of somatic mutations in ALS.
- Oliver J. Ziff
- , Jacob Neeves
- & Rickie Patani
-
Article
| Open Access T cell responses at diagnosis of amyotrophic lateral sclerosis predict disease progression
T cell responses at diagnosis of amyotrophic lateral sclerosis predict disease progression
Amyotrophic lateral sclerosis (ALS) is a primary neurodegenerative disease, which is characterized by increased immune cell infiltration of the central nervous system. Here authors show that the phenotypic profile of T cells in the blood and cerebrospinal fluid of newly diagnosed ALS patients can predict disease progression, thus providing evidence that T cells contribute to disease pathology.
- Solmaz Yazdani
- , Christina Seitz
- & Fang Fang
-
Article
| Open Access Stress induced TDP-43 mobility loss independent of stress granules
Stress induced TDP-43 mobility loss independent of stress granules
Amyotrophic Lateral Sclerosis related TDP-43 protein translocates to stress granules with a concomitant reduction in mobility. Here, the authors use single molecule tracking and find a stress-induced reduction in TDP-43 mobility also in the cytoplasm potentially relevant for TDP-43 aggregation.
- Lisa Streit
- , Timo Kuhn
- & Karin M. Danzer
-
Article
| Open Access NUP62 localizes to ALS/FTLD pathological assemblies and contributes to TDP-43 insolubility
NUP62 localizes to ALS/FTLD pathological assemblies and contributes to TDP-43 insolubility
ALS and FTLD are both characterized by insoluble cytoplasmic depositions of TDP43. Here the authors show that the nucleopore protein NUP62 is mislocalized in C9orf72 and sporadic ALS/FTLD and propose that it interacts with TDP-43 to promote its insolubility.
- Amanda M. Gleixner
- , Brandie Morris Verdone
- & Christopher J. Donnelly
-
Article
| Open Access Spelling interface using intracortical signals in a completely locked-in patient enabled via auditory neurofeedback training
Spelling interface using intracortical signals in a completely locked-in patient enabled via auditory neurofeedback training
The authors record neural firing rates in a patient with ALS in completely locked-in state and show that the patient can modulate neural firing rates based on auditory feedback to select letters to form words and phrases to communicate his needs and experiences.
- Ujwal Chaudhary
- , Ioannis Vlachos
- & Niels Birbaumer
-
Article
| Open Access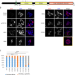 Sirtuin-1 sensitive lysine-136 acetylation drives phase separation and pathological aggregation of TDP-43
Sirtuin-1 sensitive lysine-136 acetylation drives phase separation and pathological aggregation of TDP-43
TDP-43 is a nucleic acid binding protein, whose insoluble aggregates are neuropathological hallmarks of specific subsets of patients with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Post-translational modifications and acetylation of TDP-43 impact its interaction with RNA, its localization in the cells, and are linked to disease. Using antibodies generated against TDP-43 lysine acetylation sites, sirtuin-1 was found to potently deacetylate amber suppressed [acK136]TDP-43 and reduce its aggregation propensity. Thus, distinct lysine acetylations modulate nuclear import, RNA binding as well as phase separation and aggregation of TDP-43, suggesting regulatory mechanisms for TDP-43 pathogenesis.
- Jorge Garcia Morato
- , Friederike Hans
- & Philipp J. Kahle
-
Article
| Open Access Variant-selective stereopure oligonucleotides protect against pathologies associated with C9orf72-repeat expansion in preclinical models
Variant-selective stereopure oligonucleotides protect against pathologies associated with C9orf72-repeat expansion in preclinical models
C9orf72 expansion mutations are the most common genetic cause of ALS and FTD, which have limited therapies. The authors generate stereopure oligonucleotides that selectively deplete expansion-containing transcripts and protect against expansion-associated pathologies in preclinical models.
- Yuanjing Liu
- , Jean-Cosme Dodart
- & Robert H. Brown Jr.
-
Article
| Open Access Bi-allelic variants in RNF170 are associated with hereditary spastic paraplegia
Bi-allelic variants in RNF170 are associated with hereditary spastic paraplegia
Disturbances in IP3 receptor-mediated release of Ca2+ from the endoplasmatic reticulum are associated with neurodegenerative disease. Here, the authors identify in four families with hereditary spastic paraplegia biallelic mutations in RNF170 that associate with increased basal levels of IP3 receptors.
- Matias Wagner
- , Daniel P. S. Osborn
- & Rebecca Schüle
-
Article
| Open Access First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound
First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound
MR-focused ultrasound can be used to transiently open the blood-brain barrier (BBB). Here, the authors report the results of a first-in-human trial on four patients with amyotrophic lateral sclerosis (ALS), showing that the procedure reversibly permeabilised the BBB in the motor cortex without complications, and suggest that the procedure could in the future be used to increase drug delivery in ALS patients.
- Agessandro Abrahao
- , Ying Meng
- & Lorne Zinman
-
Article
| Open Access Modulation of actin polymerization affects nucleocytoplasmic transport in multiple forms of amyotrophic lateral sclerosis
Modulation of actin polymerization affects nucleocytoplasmic transport in multiple forms of amyotrophic lateral sclerosis
Although defects in nucleocytoplasmic transport (NCT) may be central to the pathogenesis of ALS, the molecular mechanisms modulating the nuclear pore function are still largely unknown. Here, authors show that genetic and pharmacological modulation of actin polymerization disrupts nuclear pore integrity and can be targeted to rescue nuclear pore instability and dysfunction caused by mutant PFN1 as well as by C9ORF72 repeat expansion
- Anthony Giampetruzzi
- , Eric W. Danielson
- & Claudia Fallini
-
Article
| Open Access A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy
A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy
Many Amyotrophic Lateral Sclerosis (ALS)-linked mutations cause accumulation of stress granules, and most ALS cases are caused by repeat expansions in C9ORF72. Here the authors show that C9ORF72 and the autophagy receptor p62 interact to associate with proteins symmetrically dimethylated on arginines such as FUS, to eliminate stress granules by autophagy.
- Maneka Chitiprolu
- , Chantal Jagow
- & Derrick Gibbings
-
Article
| Open Access A small-molecule inhibitor of SOD1-Derlin-1 interaction ameliorates pathology in an ALS mouse model
A small-molecule inhibitor of SOD1-Derlin-1 interaction ameliorates pathology in an ALS mouse model
Amyotrophic lateral sclerosis (ALS) is a neurological disease that leads to loss of voluntary muscle movement. Here, the authors screen for molecules that disrupt interaction between SOD1, a protein linked to ALS, and Derlin-1, and find an inhibitor that reduces pathology in an ALS mouse model.
- Naomi Tsuburaya
- , Kengo Homma
- & Hidenori Ichijo
-
Article
| Open Access CUG initiation and frameshifting enable production of dipeptide repeat proteins from ALS/FTD C9ORF72 transcripts
CUG initiation and frameshifting enable production of dipeptide repeat proteins from ALS/FTD C9ORF72 transcripts
Repeat-associated non-AUG (RAN) translation contributes to the pathogenic mechanism of several microsatellite expansion diseases. Here the authors delineate the different steps involved in recruiting the ribosome to initiate G4C2 RAN translation to produce poly-Glycine Alanine, poly-Glycine Proline, and poly-Glycine Arginine repeats.
- Ricardos Tabet
- , Laure Schaeffer
- & Clotilde Lagier-Tourenne
-
Article
| Open Access Cross-ethnic meta-analysis identifies association of the GPX3-TNIP1 locus with amyotrophic lateral sclerosis
Cross-ethnic meta-analysis identifies association of the GPX3-TNIP1 locus with amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a rapidly progressing neurodegenerative disease. Here, Wray and colleagues identify association of the GPX3-TNIP1 locus with ALS using cross-ethnic meta-analyses.
- Beben Benyamin
- , Ji He
- & Dongsheng Fan
-
Article
| Open Access Genetic correlation between amyotrophic lateral sclerosis and schizophrenia
Genetic correlation between amyotrophic lateral sclerosis and schizophrenia
Relatives of patients with amyotrophic lateral sclerosis have an unexpectedly high incidence of schizophrenia. Here, the authors show a genetic link between the two conditions, suggesting shared neurobiological mechanisms.
- Russell L. McLaughlin
- , Dick Schijven
- & Michael C. O’Donovan
-
Article
| Open Access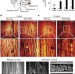 Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging
Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging
Sensitive and label-free imaging methods to visualize nerve degeneration are currently lacking. Here authors show that stimulated Raman scattering (SRS) microscopy can be used to monitor peripheral nerve degeneration in mouse models of amyotrophic lateral sclerosis (ALS) and in postmortem tissue from ALS patients.
- Feng Tian
- , Wenlong Yang
- & Kevin Eggan
-
Article
| Open Access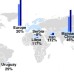 Projected increase in amyotrophic lateral sclerosis from 2015 to 2040
Projected increase in amyotrophic lateral sclerosis from 2015 to 2040
The socioeconomic burden of amyotrophic lateral sclerosis (ALS) is high, but the projected number of cases in the upcoming years is unclear. Here, the authors estimate the number and distribution of ALS cases to 2040, and show that cases are projected to increase, particularly in developing nations.
- Karissa C. Arthur
- , Andrea Calvo
- & Bryan J. Traynor
-
Article
| Open Access Caloric restriction blocks neuropathology and motor deficits in Machado–Joseph disease mouse models through SIRT1 pathway
Caloric restriction blocks neuropathology and motor deficits in Machado–Joseph disease mouse models through SIRT1 pathway
SIRTs have been reported to provide neuroprotective actions in polyglutamine diseases, and are linked to the beneficial effects of caloric restrictive diets. Here, the authors show caloric restriction improves behavioural and neuropathological deficits in MJD model mice, an effect dependent on SIRT1 activity.
- Janete Cunha-Santos
- , Joana Duarte-Neves
- & Cláudia Cavadas
-
Article
| Open Access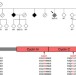 CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia
CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia
Ian Blair and colleagues use genome-wide linkage analysis and whole exome sequencing to identify mutations in the CCNF gene in large cohorts of amyotrophic lateral sclerosis and frontotemporal dementia patients. In addition to validating the mutations in international cohorts, the authors also show that mutant CCNFgene product affects ubiquitination and protein degradation in cultured cells.
- Kelly L. Williams
- , Simon Topp
- & Ian P. Blair
-
Article
| Open Access ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function
ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function
The mechanism by which FUS mutations cause familial ALS remains unclear. Here, the authors use mouse transgenic models to show that a toxic gain-of-function underlies motor neuron degeneration, and that the toxicity of mutant FUS does not depend on a loss or excess of FUS activity.
- Aarti Sharma
- , Alexander K. Lyashchenko
- & Neil A. Shneider
-
Article
| Open Access Direct optical activation of skeletal muscle fibres efficiently controls muscle contraction and attenuates denervation atrophy
Direct optical activation of skeletal muscle fibres efficiently controls muscle contraction and attenuates denervation atrophy
Nerve damage can lead to skeletal muscle paralysis and atrophy. Here, the authors show that localized photostimulation of mouse calf muscle expressing the light-sensitive channel Channelrhodopsin-2 generates contraction in the absence of neural impulses and prove that this strategy can be used to prevent muscle atrophy.
- Philippe Magown
- , Basavaraj Shettar
- & Victor F. Rafuse
-
Article |
 Neurodegeneration in C. elegans models of ALS requires TIR-1/Sarm1 immune pathway activation in neurons
Neurodegeneration in C. elegans models of ALS requires TIR-1/Sarm1 immune pathway activation in neurons
Abnormal accumulation of TDP-43 and FUS proteins is found in a neurodegenerative disease amyotrophic lateral sclerosis. Here the authors show by modelling the disease in worms that these proteins activate local and distal immune responses, and blocking this pathway in neurons ameliorates the disease.
- Julie Vérièpe
- , Lucresse Fossouo
- & J Alex Parker
-
Article
| Open Access Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression
Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression
Perineuronal astrocyte reactivity is implicated in functional recovery following nerve injury but exactly how this happens is unclear. Tyzack et al. show that perineuronal astrocytes facilitate the recovery of synaptic inputs to damaged neurons via STAT3-dependent upregulation of the astrocytic protein TSP-1.
- Giulia E. Tyzack
- , Sergey Sitnikov
- & András Lakatos

 Mutant GGGGCC RNA prevents YY1 from binding to Fuzzy promoter which stimulates Wnt/β-catenin pathway in C9ALS/FTD
Mutant GGGGCC RNA prevents YY1 from binding to Fuzzy promoter which stimulates Wnt/β-catenin pathway in C9ALS/FTD