Article
|
Open Access
Featured
-
-
Article
| Open Access Cryo-EM structures reveal how phosphate release from Arp3 weakens actin filament branches formed by Arp2/3 complex
Cryo-EM structures reveal how phosphate release from Arp3 weakens actin filament branches formed by Arp2/3 complex
Arp2/3 complex forms branched actin filaments for cell movements. Here, the authors report cryo-EM structures of branch junctions with ADP or ADPBeFx (to mimic γ-phosphate) bound to Arp3 to explain why γ-phosphate dissociation destabilizes branches.
- Sai Shashank Chavali
- , Steven Z. Chou
- & Charles V. Sindelar
-
Article
| Open Access A structural and dynamic visualization of the interaction between MAP7 and microtubules
A structural and dynamic visualization of the interaction between MAP7 and microtubules
Integrated structural data show that the MAP7 microtubule binding domain stabilizes the microtubule lattice through binding along protofilaments. Both strong and weak interactions between MAP7 and the lattice extend beyond a single tubulin dimer and include the tubulin C-terminal tails.
- Agnes Adler
- , Mamata Bangera
- & Marc Baldus
-
Article
| Open Access Dynamic interactions between E-cadherin and Ankyrin-G mediate epithelial cell polarity maintenance
Dynamic interactions between E-cadherin and Ankyrin-G mediate epithelial cell polarity maintenance
The maintenance of cell polarity depends on adhesion complexes that tether to the cytoskeleton. Here the authors show the dynamic nature of E-cadherin–Ankyrin-G complex formation and investigate its functional role in epithelial cell polarity maintenance.
- Chao Kong
- , Xiaozhan Qu
- & Chao Wang
-
Article
| Open Access Structural insights into the mechanism of GTP initiation of microtubule assembly
Structural insights into the mechanism of GTP initiation of microtubule assembly
In this study the authors explore the enigma of GTP-triggered microtubule assembly. The proposed flexible model emphasizes longitudinal and lateral contacts, enhancing our understanding of microtubule nucleation and assembly.
- Ju Zhou
- , Anhui Wang
- & Hong-Wei Wang
-
Article
| Open Access Control of motor landing and processivity by the CAP-Gly domain in the KIF13B tail
Control of motor landing and processivity by the CAP-Gly domain in the KIF13B tail
Intracellular transport of material along microtubules by kinesin motors is critical for cellular homeostasis. Here the authors uncover a unique role for a specialized kinesin tail domain in directing motor transport along specific microtubule tracks.
- Xiangyu Fan
- & Richard J. McKenney
-
Article
| Open Access Structural basis of protein condensation on microtubules underlying branching microtubule nucleation
Structural basis of protein condensation on microtubules underlying branching microtubule nucleation
TPX2 is a key factor stimulating branching microtubule (MT) nucleation. TPX2 forms condensates on MTs critical for branching. In this work, the authors report the atomic-level structure of TPX2 C-terminal minimal active domain on MT lattice and its binding interface, determined by magic-angle-spinning NMR.
- Changmiao Guo
- , Raymundo Alfaro-Aco
- & Tatyana Polenova
-
Comment
| Open Access Structural insights into how augmin augments the mitotic spindle
Structural insights into how augmin augments the mitotic spindle
Cell division critically requires amplification of microtubules (MTs) in the bipolar mitotic spindle. This relies on the filamentous augmin complex that enables MT branching. Studies by Gabel et al., Zupa et al. and Travis et al. describe consistent integrated atomic models of the extraordinarily flexible augmin complex. Their work prompts the question: what is this flexibility really needed for?
- Szymon W. Manka
-
Article
| Open Access Integrated model of the vertebrate augmin complex
Integrated model of the vertebrate augmin complex
Many microtubules in the mitotic spindle are made through microtubule branching. Here, the authors report a structural model of the augmin complex and insights into its role in microtubule branching.
- Sophie M. Travis
- , Brian P. Mahon
- & Sabine Petry
-
Article
| Open Access Watching the release of a photopharmacological drug from tubulin using time-resolved serial crystallography
Watching the release of a photopharmacological drug from tubulin using time-resolved serial crystallography
Photopharmacology manipulates the biological activity of small molecules by light. Using an X-ray laser, the authors follow the release of the drug azo-combretastatin A4 from tubulin and the concomitant structural changes over nine orders of magnitude in time.
- Maximilian Wranik
- , Tobias Weinert
- & Jörg Standfuss
-
Article
| Open Access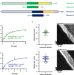 Mechanochemical tuning of a kinesin motor essential for malaria parasite transmission
Mechanochemical tuning of a kinesin motor essential for malaria parasite transmission
Plasmodium kinesin-8B is essential for male gamete formation and its absence blocks parasite transmission. Using cryo-EM and TIRF, the authors report how kinesin-8B motor domains are tuned to support microtubule motility and depolymerase activity.
- Tianyang Liu
- , Fiona Shilliday
- & Carolyn A. Moores
-
Article
| Open Access F-actin architecture determines constraints on myosin thick filament motion
F-actin architecture determines constraints on myosin thick filament motion
While the impact of F-actin architecture on stress transmission is well studied, the role of architecture on stress generation remains unclear. Here authors use in vitro model and show that distinct organizations constrain myosin motion.
- Camelia G. Muresan
- , Zachary Gao Sun
- & Michael P. Murrell
-
Article
| Open Access Magic-angle-spinning NMR structure of the kinesin-1 motor domain assembled with microtubules reveals the elusive neck linker orientation
Magic-angle-spinning NMR structure of the kinesin-1 motor domain assembled with microtubules reveals the elusive neck linker orientation
In this work the authors report the structure of nucleotide-free kinesin-1 motor domain (apo-KIF5B) in complex with paclitaxel-stabilized microtubules using magic-angle-spinning (MAS) NMR spectroscopy. The study provides insights into the dynamic changes under which the neck linker goes upon binding to ADP.
- Chunting Zhang
- , Changmiao Guo
- & Tatyana Polenova
-
Article
| Open Access Competing instabilities reveal how to rationally design and control active crosslinked gels
Competing instabilities reveal how to rationally design and control active crosslinked gels
This paper investigates the origin of two distinct instabilities in active gels of biopolymers and molecular motors. Combining experiments and theory, it shows how to rationally design and control active materials with targeted elasticity and activity.
- Bibi Najma
- , Minu Varghese
- & Guillaume Duclos
-
Article
| Open Access The augmin complex architecture reveals structural insights into microtubule branching
The augmin complex architecture reveals structural insights into microtubule branching
The formation of branched microtubule networks in mitotic spindles depends on the augmin complex. Zupa, Würtz et al. elucidate the molecular architecture and conformational plasticity of the augmin complex using integrative structural biology, providing structural insights into microtubule branching.
- Erik Zupa
- , Martin Würtz
- & Stefan Pfeffer
-
Article
| Open Access Structures reveal a key mechanism of WAVE regulatory complex activation by Rac1 GTPase
Structures reveal a key mechanism of WAVE regulatory complex activation by Rac1 GTPase
Rho-family GTPase Rac1 activates the WAVE complex (WRC) to promote Arp2/3-mediated actin assembly in various processes. Here, the authors determined cryo-EM structures of WRC bound to Rac1 in different states, revealing how Rac1 binding activates WRC.
- Bojian Ding
- , Sheng Yang
- & Baoyu Chen
-
Article
| Open Access Molecular architecture of the augmin complex
Molecular architecture of the augmin complex
The eight-subunit augmin complex is required to nucleate branching microtubules and create a robust mitotic spindle during cell division. Here, the authors use cryo-EM, crosslinking mass spectrometry, and computational tools to build a structural model of the human augmin complex.
- Clinton A. Gabel
- , Zhuang Li
- & Leifu Chang
-
Article
| Open Access Multistep orthophosphate release tunes actomyosin energy transduction
Multistep orthophosphate release tunes actomyosin energy transduction
Release of the ATP hydrolysis product orthophosphate (Pi) from the myosin active site is central in force generation but is poorly understood. Here, Moretto et al. present evidence for multistep Pi-release reconciling apparently contradictory results.
- Luisa Moretto
- , Marko Ušaj
- & Alf Månsson
-
Article
| Open Access Kinesin-8-specific loop-2 controls the dual activities of the motor domain according to tubulin protofilament shape
Kinesin-8-specific loop-2 controls the dual activities of the motor domain according to tubulin protofilament shape
Kinesin-8s are dual-activity motor proteins that can move processively on microtubules and depolymerize microtubule plus-ends. This study shows how kinesin-8s alternate between a promotility and a pro-microtubule-depolymerization state via their tubulin shape-sensing loop-2 region.
- Byron Hunter
- , Matthieu P. M. H. Benoit
- & John S. Allingham
-
Article
| Open Access In vitro reconstitution of Escherichia coli divisome activation
In vitro reconstitution of Escherichia coli divisome activation
In E. coli, FtsA and FtsZ control the place and time of cell division. Here, the authors use in vitro experiments to show how FtsA can follow FtsZ treadmilling and that downstream proteins form dynamic copolymers with FtsA to initiate division.
- Philipp Radler
- , Natalia Baranova
- & Martin Loose
-
Article
| Open Access Magic angle spinning NMR structure of human cofilin-2 assembled on actin filaments reveals isoform-specific conformation and binding mode
Magic angle spinning NMR structure of human cofilin-2 assembled on actin filaments reveals isoform-specific conformation and binding mode
Despite their relevance as regulators of actin severing and filament disassembly, few structural insights into the mechanism of cofilin-isoform-specific severing activity are reported. Here, the authors provide structural insights towards actin severing activity by human cofilin-2 obtained by MAS NMR and all-atom MD simulations. The results reveal an isoform-specific binding mode unique to CFL2 that may be related to its potent severing properties in-vivo.
- Jodi Kraus
- , Ryan W. Russell
- & Tatyana Polenova
-
Article
| Open Access Molecular mechanism of Arp2/3 complex inhibition by Arpin
Molecular mechanism of Arp2/3 complex inhibition by Arpin
The Arp2/3 complex inhibitor Arpin controls cell migration by interrupting a feedback loop involving Rac-WAVE-Arp2/3 complex Here, the authors use structural, biochemical, and cellular studies to reveal Arpin’s mechanism of inhibition.
- Fred E. Fregoso
- , Trevor van Eeuwen
- & Roberto Dominguez
-
Article
| Open Access Targeting the actin nucleation promoting factor WASp provides a therapeutic approach for hematopoietic malignancies
Targeting the actin nucleation promoting factor WASp provides a therapeutic approach for hematopoietic malignancies
Cancer cells proliferate and invade via cytoskeletal proteins such as WASp, exclusively expressed in hematopoietic cells. Here the authors show a specific small molecule compound inhibiting cancer cell activity by WASp degradation and demonstrating its therapeutic potential in vitro and in vivo.
- Guy Biber
- , Aviad Ben-Shmuel
- & Mira Barda-Saad
-
Article
| Open Access A barbed end interference mechanism reveals how capping protein promotes nucleation in branched actin networks
A barbed end interference mechanism reveals how capping protein promotes nucleation in branched actin networks
The assembly of branched actin networks depends on the heterodimeric capping protein CP/CapZ. Combining cryoEM, in vitro reconstitution and cell biological assays, the authors show that CP not only prevents actin filament elongation but also selectively masks actin filament ends to promote nucleation.
- Johanna Funk
- , Felipe Merino
- & Peter Bieling
-
Article
| Open Access Measuring expression heterogeneity of single-cell cytoskeletal protein complexes
Measuring expression heterogeneity of single-cell cytoskeletal protein complexes
Existing methods for multimeric protein complex quantification in single cells suffer from limited selectivity and sensitivity. Here the authors report Single-cell protein Interaction Fractionation Through Electrophoresis and immunoassay Readout (SIFTER) and use this to probe the effects of cellular stress.
- Julea Vlassakis
- , Louise L. Hansen
- & Amy E. Herr
-
Article
| Open Access Disrupting the LINC complex by AAV mediated gene transduction prevents progression of Lamin induced cardiomyopathy
Disrupting the LINC complex by AAV mediated gene transduction prevents progression of Lamin induced cardiomyopathy
Mutations in the LaminA gene are the second most common inherited cause of Dilated Cardiomyopathy, a major form of heart failure. Here the authors show that disruption of the nuclear protein SUN1 in cardiomyocytes, by AAV mediated transduction of a SUN1 inhibitor, significantly suppress cardiomyopathy progression, providing a potential therapeutic route to treat this disease.
- Ruth Jinfen Chai
- , Hendrikje Werner
- & Colin L. Stewart
-
Article
| Open Access Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors
Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors
Mitochondrial transport toward both the plus- and minus-ends of microtubules is mediated by motor proteins linked to mitochondria by TRAK adaptor proteins. Here the authors investigate the role of TRAK2 as a bidirectional motor adaptor, and propose a model where TRAK2 coordinates the activities of opposing kinesin-1 and cytoplasmic dynein motors as a single interdependent motor complex.
- Adam R. Fenton
- , Thomas A. Jongens
- & Erika L. F. Holzbaur
-
Article
| Open Access KRAP tethers IP3 receptors to actin and licenses them to evoke cytosolic Ca2+ signals
KRAP tethers IP3 receptors to actin and licenses them to evoke cytosolic Ca2+ signals
Calcium signals initiated by IP3 receptors in ER membranes regulate most cellular activities. Here, the authors show that KRas-induced actininteracting protein (KRAP) tethers a small subset of IP3 receptors to actin and licenses them to evoke cytosolic calcium signals.
- Nagendra Babu Thillaiappan
- , Holly A. Smith
- & Colin W. Taylor
-
Article
| Open Access Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments
Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments
Intracellular trafficking of organelles is driven by kinesin-1 stepping along microtubules, but crowding conditions impede kinesin-1 motility. Here authors demonstrate that TRAK1, an adaptor protein essential for mitochondrial trafficking, activates kinesin-1 and increases robustness of kinesin-1 stepping on crowded microtubule surfaces.
- Verena Henrichs
- , Lenka Grycova
- & Zdenek Lansky
-
Article
| Open Access Essential dynamic interdependence of FtsZ and SepF for Z-ring and septum formation in Corynebacterium glutamicum
Essential dynamic interdependence of FtsZ and SepF for Z-ring and septum formation in Corynebacterium glutamicum
The mechanisms of Z-ring assembly and regulation in bacteria are poorly understood, particularly in non-model organisms. Here, Sogues et al. study the interaction between FtsZ and SepF in Corynebacterium glutamicum, showing an essential interdependence of these proteins for formation of a functional Z-ring.
- Adrià Sogues
- , Mariano Martinez
- & Pedro M. Alzari
-
Article
| Open Access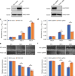 Quantitative SUMO proteomics identifies PIAS1 substrates involved in cell migration and motility
Quantitative SUMO proteomics identifies PIAS1 substrates involved in cell migration and motility
PIAS1 is an E3 SUMO ligase involved in various cellular processes. Here, the authors use quantitative proteomics to identify potential PIAS1 substrates in human cells and elucidate the biological consequences of PIAS1-mediated SUMOylation of vimentin.
- Chongyang Li
- , Francis P. McManus
- & Pierre Thibault
-
Article
| Open Access Direct observation of dynamic protein interactions involving human microtubules using solid-state NMR spectroscopy
Direct observation of dynamic protein interactions involving human microtubules using solid-state NMR spectroscopy
Microtubule (MT) organization is regulated by many microtubule-associated proteins (MAPs) that contain intrinsically disordered regions. Here authors produce [13C, 15N] labeled, functional microtubules from human cells for solid-state NMR which allows studying MAP-MT interactions.
- Yanzhang Luo
- , ShengQi Xiang
- & Marc Baldus
-
Article
| Open Access Mechanism of synergistic actin filament pointed end depolymerization by cyclase-associated protein and cofilin
Mechanism of synergistic actin filament pointed end depolymerization by cyclase-associated protein and cofilin
The cofilin family proteins are actin disassembly factors but the disassembly mechanism is poorly understood. Here authors show that cyclase-associated-protein (CAP) works in synergy with cofilin to accelerate actin filament depolymerization by nearly 100-fold and reveal how CAP destabilizes the interface between terminal actin subunits.
- Tommi Kotila
- , Hugo Wioland
- & Pekka Lappalainen
-
Article
| Open Access Polarisome scaffolder Spa2-mediated macromolecular condensation of Aip5 for actin polymerization
Polarisome scaffolder Spa2-mediated macromolecular condensation of Aip5 for actin polymerization
The polarisome is a dynamic protein complex that nucleates F-actin for polarized yeast growth, but its regulation is unclear. Here, the authors report that the polarisome protein Aip5 undergoes Spa2-mediated phase separation in physiological and stress conditions, potentially for regulating actin assembly.
- Ying Xie
- , Jialin Sun
- & Yansong Miao
-
Article
| Open Access Lamin A molecular compression and sliding as mechanisms behind nucleoskeleton elasticity
Lamin A molecular compression and sliding as mechanisms behind nucleoskeleton elasticity
Lamin A is critical for nuclear architecture but its structure and assembly are not fully understood. Here, the authors use quantitative cross-linking mass spectrometry to map intra- and intermolecular interactions within lamin homomers, providing insights into the molecular basis for lamin’s mechanical properties.
- Alex A. Makarov
- , Juan Zou
- & Eric C. Schirmer
-
Article
| Open Access PTPN21 and Hook3 relieve KIF1C autoinhibition and activate intracellular transport
PTPN21 and Hook3 relieve KIF1C autoinhibition and activate intracellular transport
The kinesin-3 KIF1C transports dense core vesicles in neurons and delivers integrins to cell adhesions sites. Here the authors show that KIF1C's autoinhibitory interactions are released upon binding of protein tyrosine phosphatase PTPN21 or cargo adapter Hook3 resulting in cargo-activated transport.
- Nida Siddiqui
- , Alexander James Zwetsloot
- & Anne Straube
-
Article
| Open Access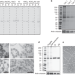 The dynamic and structural properties of axonemal tubulins support the high length stability of cilia
The dynamic and structural properties of axonemal tubulins support the high length stability of cilia
The axoneme in cilia and flagella has exceptionally high stability despite being composed of microtubules that are known to be highly dynamic. Here authors extract tubulin from different components of Chlamydomonas reinhardtii axonemes and characterize their properties.
- Ron Orbach
- & Jonathon Howard
-
Article
| Open Access A cysteine-based molecular code informs collagen C-propeptide assembly
A cysteine-based molecular code informs collagen C-propeptide assembly
Collagen proteins assemble into trimers from distinct monomers with high specificity, yet the molecular basis for this specificity remains unclear. Here the authors demonstrate the crucial role of conserved C-terminal domain cysteine residues and calcium in homotrimeric procollagen assembly.
- Andrew S. DiChiara
- , Rasia C. Li
- & Matthew D. Shoulders
-
Article
| Open Access Abp1 promotes Arp2/3 complex-dependent actin nucleation and stabilizes branch junctions by antagonizing GMF
Abp1 promotes Arp2/3 complex-dependent actin nucleation and stabilizes branch junctions by antagonizing GMF
Abp1, a type II actin nucleation promoting factor, is a known component of branched actin networks but its mechanism remains poorly understood. Here, the authors find that Abp1 enhances Arp2/3-mediated actin branch formation, and blocks ‘debranching’ by GMF, making it a pro-branching factor.
- Siyang Guo
- , Olga S. Sokolova
- & Bruce L. Goode
-
Article
| Open Access Ternary complex of Kif2A-bound tandem tubulin heterodimers represents a kinesin-13-mediated microtubule depolymerization reaction intermediate
Ternary complex of Kif2A-bound tandem tubulin heterodimers represents a kinesin-13-mediated microtubule depolymerization reaction intermediate
The kinesin-13 family of microtubule (MT) depolymerases are major regulators of MT dynamics. Here the authors provide insights into the MT depolymerization mechanism by solving the crystal structure of a kinesin-13 monomer (Kif2A) in complex with two bent αβ-tubulin heterodimers.
- Daria Trofimova
- , Mohammadjavad Paydar
- & John S. Allingham
-
Article
| Open Access Structural basis for cofilin binding and actin filament disassembly
Structural basis for cofilin binding and actin filament disassembly
Cofilin is a small actin-binding protein that accelerates actin turnover by disassembling actin filaments. Here the authors present the 3.8 Å cryo-EM structure of a cofilin-decorated actin filament and discuss mechanistic implications.
- Kotaro Tanaka
- , Shuichi Takeda
- & Akihiro Narita
-
Article
| Open Access Competition between microtubule-associated proteins directs motor transport
Competition between microtubule-associated proteins directs motor transport
Motor and non-motor microtubule-associated proteins (MAPs) bind to the microtubule lattice, but it is unclear how their binding activities are coordinated and how this impacts motor transport. Here the authors show how MAP competition controls microtubule access to determine the distribution and balance of motor activity.
- Brigette Y. Monroy
- , Danielle L. Sawyer
- & Kassandra M. Ori-McKenney
-
Article
| Open Access Lymphocyte-specific protein 1 regulates mechanosensory oscillation of podosomes and actin isoform-based actomyosin symmetry breaking
Lymphocyte-specific protein 1 regulates mechanosensory oscillation of podosomes and actin isoform-based actomyosin symmetry breaking
The actomyosin cytoskeleton plays an important role in polarised cell migration. Here the authors identify lymphocyte-specific protein (LSP)-1 as a regulator of actomyosin contractility in macrophages, by competing with supervillin for myosin IIA activators acting specifically on the β-actin isoform.
- Pasquale Cervero
- , Christiane Wiesner
- & Stefan Linder
-
Article
| Open Access Catastrophic disassembly of actin filaments via Mical-mediated oxidation
Catastrophic disassembly of actin filaments via Mical-mediated oxidation
MICAL Redox enzymes post-translationally modify F-actin to promote its cellular destabilization. Here, the authors present a 3.9Å cryoEM structure of Mical-oxidized F-actin, showing its nucleotide-state dependent dynamic instability and susceptibility to cofilin-induced severing in the presence of inorganic phosphate.
- Elena E. Grintsevich
- , Peng Ge
- & Emil Reisler
-
Article
| Open Access Nucleotide– and Mal3-dependent changes in fission yeast microtubules suggest a structural plasticity view of dynamics
Nucleotide– and Mal3-dependent changes in fission yeast microtubules suggest a structural plasticity view of dynamics
Microtubules are vital and highly conserved components of the cytoskeleton. Here the authors carry out a structural analysis of fission yeast microtubules in the presence and absence of the microtubule end-binding protein Mal3 that demonstrates structural plasticity amongst microtubule polymers.
- Ottilie von Loeffelholz
- , Neil A. Venables
- & Carolyn A. Moores
-
Article
| Open Access NDP52 activates nuclear myosin VI to enhance RNA polymerase II transcription
NDP52 activates nuclear myosin VI to enhance RNA polymerase II transcription
Myosin VI (MVI) is known to interact with RNA Polymerase II and to play non-cytoplasmic roles in cells. Here, the authors provide evidence that the transcription co-activator NDP52 regulates MVI binding to DNA and that MVI interacts with nuclear receptors to drive gene expression.
- Natalia Fili
- , Yukti Hari-Gupta
- & Christopher P. Toseland
-
Article
| Open Access Subnanometre-resolution structure of the doublet microtubule reveals new classes of microtubule-associated proteins
Subnanometre-resolution structure of the doublet microtubule reveals new classes of microtubule-associated proteins
Cilia are hair-like appendages involved in cell motility and sensory reception. Here, the authors report a high resolution cryo-EM structure of the microtubule doublet from motile cilia and identify microtubule inner proteins (MIPs) bound to the inner surface of the doublet that appear to stabilize its structure.
- Muneyoshi Ichikawa
- , Dinan Liu
- & Khanh Huy Bui
-
Article
| Open Access Identification of an ATP-controlled allosteric switch that controls actin filament nucleation by Arp2/3 complex
Identification of an ATP-controlled allosteric switch that controls actin filament nucleation by Arp2/3 complex
Arp2/3 complex nucleates branched actin filaments, and is inactive in the absense of activators. Here the authors present a model of Arp2/3 autoinhibition, whereby the Arp3 C-terminal tail acts as a structural switch that blocks movement of Arp2 and Arp3 into an activated filament-like conformation.
- Max Rodnick-Smith
- , Su-Ling Liu
- & Brad J. Nolen
-
Article
| Open Access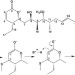 Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule
Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule
Microtubule assembly and disassembly is the target of many anticancer therapies, with β-tubulin the most-frequent target. Here, the authors used biochemical and biophysical techniques to demonstrate pironetin binds to α-tubulin and thereby inhibits microtubule polymerization providing a basis for the rational design of novel anticancer drugs.
- Jianhong Yang
- , Yuxi Wang
- & Lijuan Chen
-
Article
| Open Access Tum/RacGAP functions as a switch activating the Pav/kinesin-6 motor
Tum/RacGAP functions as a switch activating the Pav/kinesin-6 motor
Centralspindlin consists of dimeric kinesin-6 and dimeric RacGAP, and is involved in the organization of anaphase midzone microtubules. Here, the authors show that the RacGAP is needed for motor activity at the plus-end of microtubules, but not for the bundling activity associated with kinesin-6.
- Li Tao
- , Barbara Fasulo
- & William Sullivan

 F-actin architecture determines the conversion of chemical energy into mechanical work
F-actin architecture determines the conversion of chemical energy into mechanical work