A young woman with wavy brown hair and maroon nails lay on a gurney in a hospital room in Copenhagen. Her extended left arm was wired with electrodes. A pop pierced the air every few seconds—an electric shock. Each time, the woman’s fingers twitched. She winced. She was to receive hundreds of shocks that day.
The woman, attended to by several physicians in laboratory coats, was renting out her arm for 1,000 Danish kroner, about $187. Thomas Heimburg, a physicist trained in quantum mechanics and biophysics, sat on a stool, safely out of the way, sketching on his iPad the details of a harsh experiment that he hoped would produce profound results.
The physicians had injected the woman’s arm with the anesthetic lidocaine—a dose strong enough to deaden her limb for surgery. At first, the nerves in her arm did not respond to the shocks. But the attendants gradually dialed up the current. At this moment, the jolts were 40 milliamperes, nearly 10 times their original strength—similar to the electricity coursing through a five-watt lightbulb.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Pop—another shock. The woman’s hand twitched like a dying snake. Heimburg paid no notice as he stared at a computer monitor on the wall. A waveform depicting the electric signal in the arm muscle and nerve leaped across the screen in one large spike—evidence that the ever increasing shocks had started to overcome the anesthetic. The nerve was now firing as strongly as it did before the woman was anesthetized. Heimburg was pleased. “The things that are written in books,” he said quietly, “they are in contradiction to this.”
Heimburg, who works at the Niels Bohr Institute in Copenhagen, famous for physics research, hopes to contradict lots of things written in books. This experiment, which I witnessed in December 2011, was designed to investigate a long-standing medical mystery.
Physicians have administered general anesthetics for 170 years. They have discovered dozens of effective compounds. When given at progressively higher doses, the drugs all silence nerve functions in the body and brain in the same distinct order: first memory formation, then pain sensation, then consciousness, and eventually breathing. This same sequence happens across all animals, from humans to flies.
Yet no one knows how anesthesia actually works. The molecular structures of nitrous oxide, ether, sevoflurane and xenon are so different that it is unlikely they exert their common effects by binding to equivalent proteins in cells, as other drugs do.
Heimburg thinks anesthetics work in a radically different way: by changing the mechanical properties of a nerve. If that is true, it means that nerve cells, or neurons, throughout the body and brain are mechanical machines, not the electric circuits scientists have believed in for decades. In Heimburg’s view, the electric pulses are simply the side effects of a physical shock wave that ripples down the nerve, similar to the way sound waves travel. He thinks anesthetics silence nerves by soaking into the fatty membranes that encase nerve fibers, rendering them too soft to transmit the shock waves, like a guitar string too slack to twang.
It was tempting to dismiss Heimburg as nutty when I watched that experiment. But in the seven years since then, he and his colleagues have rolled out an array of evidence: delicate measurements of how mechanical waves move through single nerve cells and of how much and how quickly the membranes can expand and contract, as well as studies showing how anesthetics alter these properties. Other scientists are starting to take an interest. Now Heimburg is preparing for a crucial experiment that could clinch his case: measuring the heat emitted by a single nerve cell as a pulse shoots through it.
Heimburg’s work continues to demonstrate that the nerve pulse is more complex than most biologists may realize. The mechanical components may have been overlooked because of an accident of history: 50 years ago off-the-shelf instruments could readily measure the tiny electric impulses in neurons but not the mechanical ones. Hardware limitations influenced which discoveries scientists made and which ideas entered mainstream scientific thought. Heimburg’s experiments are now reopening a decades-old scientific schism.
The story of the mechanical neuron holds lessons for all of science about biases and accidents of history. It also could change our basic understanding of nerves, brains and intelligence. Scientists have struggled to explain how brains achieve such daunting feats as face recognition and conversation while relying on proteins in neurons that are electrically noisy and unreliable. Heimburg is showing how the mechanical waves may compensate for this noise. If his theory proves out, he could rewrite biology. Or he might just be wrong.
Hot Nerves
The neural pulse that scientists have tried for so long to explain lasts for only an instant. Step on a thumbtack, and your brain senses the pain within a fraction of a second. The signal travels through nerve fibers at up to 30 meters per second.
The fibers resemble tiny hollow pipes, finer than a hair. The pipe wall is formed by an oily cell membrane. Charged sodium and potassium atoms, called ions, hover around the inside and outside of the membrane. By the mid-1900s researchers had learned to stick electrodes into nerve cells to monitor the voltage across the membrane wall. They discovered that as a nerve pulse travels down the membrane and passes the electrode, the voltage spikes for several thousandths of a second. In 1952 two British scientists, Alan Hodgkin and Andrew Huxley, reported that the spike happens as sodium ions stream through the membrane wall from outside to inside. The voltage then reverses to normal as potassium ions gush through the membrane from inside to outside. The Hodgkin-Huxley model became the foundation of modern neurophysiology.
Hodgkin and Huxley received a Nobel Prize in 1963. But a few scientists continued to unearth observations that undermined their model, observations that Heimburg has re-created, even though some of those scientists had been written off as misguided.
Ichiji Tasaki, a senior neurobiologist at the National Institutes of Health for many years, was one of them. In 1979 he conducted an unorthodox experiment. Gazing through a microscope, he gingerly placed a fleck of shiny platinum atop a fine white thread—a nerve fiber bundle of a crab, laid bare by dissecting the animal’s leg—and trained a laser onto the platinum. By measuring the reflection of the laser light, he could detect motions that would show whether the nerve bundle briefly widened or narrowed as an electric pulse passed by. He and his then postdoctoral fellow, Kunihiko Iwasa, took hundreds of measurements. After a week, the answer was clear: every time a pulse shot through the nerve fibers, they briefly widened, then narrowed again, within a few thousandths of a second.
The ripple was minuscule: the membrane surface rose by only about seven billionths of a meter. But it coincided perfectly with the passing electric pulse, confirming a suspicion Tasaki had harbored for years: that Hodgkin and Huxley were wrong.
As far back as the 1940s, researchers had noticed that as an electric pulse passes through a nerve fiber, the translucent cell briefly becomes more opaque. By 1968 Tasaki and another team found evidence suggesting that as the pulse arrives, molecules in the membrane physically rearrange themselves, then revert to their original configuration after the pulse passes.
NEURON, or nerve cell (yellow), in the brain's hippocampus—the center of long-term memory—is supported by proteins (green and red). Credit: Robert S. McNeil Science Source
Then there was the heat. Researchers expected an electric pulse to release heat—common when electricity flows. But several teams discovered something strange. A nerve fiber’s temperature rose several millionths of a degree Celsius as a pulse raced by, yet after it passed, the temperature quickly fell again. The heat had not dissipated; instead the nerve had reabsorbed most of it, also within a few thousandths of a second. For Tasaki, the transient widening, the rearranging molecules, and the heating and cooling pointed to a startling conclusion: the nerve signal was not just a voltage pulse; it was every bit as much a mechanical pulse. Scientists who listened to nerves with electrodes were missing much of the action.
Tasaki would spend the rest of his life probing these effects. He came to believe that they originated not in the cell membrane but in a layer of protein and carbohydrate filaments just underneath it. According to his theory, as the voltage pulse arrives, the filaments absorb potassium ions and water—causing them to swell and warm—a process that then reverses itself after the pulse passes by.
As Tasaki pursued these ideas, he gradually fell out of step with the field. Other factors conspired against him. Having grown up in Japan, he spoke stilted English. “You [had] to know a lot of things to have a really substantive conversation with him,” says Peter Basser, an NIH section head in neuroscience who knew Tasaki for 20 years. “And I think a lot of people thought he wasn’t really as deep and perceptive as he was.” And although Tasaki collaborated with visiting scientists, he did not produce student protégés who would carry his ideas forward.
Emblematic of the schism was the ideological rivalry that arose between Tasaki and another prominent NIH neuroscientist, Kenneth Cole, who adhered to the mainstream view. Although the two men occupied the same lab building from the 1950s to the 1970s, they barely spoke for 15 years, except at public presentations, where one would undermine the other by standing up in the audience and posing prickly questions.
Tasaki gave up his lab during an NIH reorganization in 1997 and moved into a small space in Basser’s lab. He continued working seven days a week, well into his 90s. One day in December 2008, as he walked near his home, he lost his balance and banged his head on the ground. He died a week later at the age of 98.
By then, Tasaki’s work had disappeared from sight. “I don’t think anybody disputed that those things were being seen, because he was respected in the lab,” said Adrian Parsegian, a biophysicist at the University of Massachusetts Amherst, who was at the NIH from 1967 to 2009. Rather Tasaki’s findings “were explained away as not central” to nerve signaling—nothing more than side effects of the voltage pulse. The underlying scientific questions “didn’t get resolved,” he said. “One side got into the textbooks, and the other one didn’t.”
Fatty Liquid Becomes Crystal
Heimburg came across Tasaki’s work in the mid-1980s, while pursuing his Ph.D. at the Max Planck Institute for Biophysical Chemistry in Göttingen, Germany. Soon he found himself immersed in long sessions at the library, poring over old papers. He would eventually connect the dots in a different way than Tasaki had. He believed that the mechanical wave, the optical changes and the transient heat must occur in the fatty cell membrane of nerves throughout the body and brain, not in the protein and carbohydrate filaments below the membrane, as Tasaki had thought.
By the late 1990s Heimburg had begun doing his own experiments, compressing artificial cell membranes to see how they might respond to a mechanical shock wave. This work revealed something crucial: the membrane’s oily lipid molecules are normally fluid and randomly oriented, but they hover close to what chemists call a phase transition. Squeeze the membrane just a little bit, and the lipids condense into a highly aligned liquid crystal.
These experiments led Heimburg to declare that a nerve pulse is a mechanical shock wave that travels down the nerve membrane. As it advances, it should squeeze the membrane’s lipid molecules into a liquid crystal—a phase change that would release a small amount of heat, just as water does when it freezes. Then, as the tail end of the shock wave passes, a few thousandths of a second later, the membrane would revert to a fluid state, reabsorbing the heat. That brief transition into a liquid crystal and back would also cause the nerve membrane to widen briefly, just as Tasaki and Iwasa had seen when they shined a laser on that platinum fleck.
Heimburg’s experiments went one crucial step further. They showed how the shock wave and phase transition might be linked to the voltage spike that occurs as the pulse passes by. Heimburg found that he could push a membrane into its liquid-crystal state simply by putting it under a voltage. “People applied voltage across biologic membranes for 70 years or so, and none of these electrophysiologists had ever checked” for a liquid-crystal structure, he said.
Textbook diagrams portray cell membranes as thin, passive sheets of insulation wrapped around pipelike nerve fibers. But physicists are starting to realize that cell membranes have surprising properties. They belong to a class of materials known as piezoelectrics, which can convert mechanical forces into electric forces, and vice versa. Quartz watches run on this principle. This means that a voltage pulse traveling down a membrane will carry with it a mechanical wave. And conversely, a mechanical wave traveling down a membrane will express itself as a voltage pulse.
When Heimburg and his fellow researcher Andrew D. Jackson first published the theory in 2005, they had still never observed one of these electromechanical pulses in motion. One of Heimburg’s former students filled that gap. In 2009 Matthias Schneider, a biophysicist now at the Technical University of Dortmund in Germany, reported that he could trigger a mechanical wave by applying a voltage pulse to an artificial membrane. The pulse strength was similar to that found in nerve cells. The shock wave traveled at approximately 50 meters per second, similar to the speed at which thumbtack-triggered signals race from the foot to the brain. By 2012 Schneider had confirmed that the mechanical and voltage pulses were part of the same membrane wave.
Schneider’s most important finding came in 2014, however. A key feature of a nerve pulse is that it is all-or-nothing. If a neuron receives a weak incoming shock, it will not fire a voltage pulse. If the shock is strong enough, it will fire. “There is a threshold,” Schneider says. He found that the electromechanical waves on his artificial membranes were indeed all-or-nothing. The determining factor seemed to be whether the membrane was squished hard enough to force it into liquid-crystal form. Only then, he says, “you get a pulse.”
Anesthesia Explained
Why had Heimburg first committed to this view of nerves and anesthesia? Hoping to find out, I visited him at his office at the Niels Bohr Institute during the same week I witnessed the hospital experiment.
Heimburg had the bookshelves of a physicist, not a biologist, crammed with volumes by dead German physicists. Among them was a row of clothbound books by Hermann von Helmholtz, who in the mid-1800s formulated a key premise of thermodynamics, that energy can change form but cannot be created or destroyed. Helmholtz, incidentally, also measured the speed of nerve pulses. “I find it absolutely mandatory to read these old texts,” Heimburg said. They document the gradual discovery of fundamental connections among energy, temperature, pressure, voltage and phase transitions. These principles underlie Heimburg’s ideas about nerve function, the ideas of a physicist pushing his way into another field. “Thermodynamics is the most profound science that we have,” he said. “If you know thermodynamics, you are wise.”
He was quick to point out weaknesses in popular explanations about anesthesia. Biologists think anesthetics silence nerves by binding to and thus blocking ion channels—valves in a nerve membrane that open and close to allow sodium or potassium ions to flow through. Biologists say the flow of ions propels voltage pulses down a nerve fiber—commonly portrayed as an electric signal. But because different anesthetics have vastly different molecular structures, Heimburg could not believe they could all bind to ion channels. That explanation was “completely ridiculous,” he said, with a hint of frustration, as if pointing out something that should be obvious. Something “deeper, more profound,” must be at work.
Heimburg’s ideas were shaped in part by an old volume entitled Studien über die Narkose, or Studies of Narcosis, published by Ernest Overton in 1901. It recounts a particular experiment that caught his attention. Overton took dozens of different anesthetics and put each into a flask of water with a layer of olive oil floating on top. He shook each flask, then waited for the water and oil to separate again. He measured how much of each drug ended up in the oil versus the water. The more potent an anesthetic was in animals, the more strongly it moved into the oil, a striking result later confirmed for modern anesthetics. Olive oil and cell membranes are composed of the same oily molecules, called fatty acids. Heimburg surmised that the drugs might work by soaking into the cell membranes, altering their physical properties.
Experiments with synthetic membranes support that idea. When Heimburg infuses a membrane with an anesthetic, it prevents the membrane from becoming a liquid crystal. It does so by lowering the temperature (and raising the pressure) at which the phase transition from fluid lipid to crystalline lipid occurs—just as salt or sugar lowers the freezing point of water.
Heimburg reasoned that preventing this transition in a membrane would stop a mechanical pulse from advancing down a nerve fiber, explaining why anesthetics deaden nerves. And notably, he predicted it should be possible to overcome this effect. To create higher pressure to solidify a membrane using an electric shock, you have to crank up the current—exactly what the physicians did to the woman’s arm at the hospital in Copenhagen. Stronger electric shocks did indeed overcome the anesthetic. If anesthesia can be overcome by pushing harder on a membrane with electricity, then it should also be reversible by increasing the physical pressure on a membrane.
Biologists demonstrated this way back in 1942. They used two different anesthetics, ethanol and urethane, to inebriate tadpoles to the point that they stopped swimming. Then the scientists put the animals in a hyperbaric chamber and raised the pressure to 136 times that of the atmosphere. The anesthetic effect vanished: the tadpoles resumed swimming. When the pressure was lowered, the animals again fell motionless. “It’s very surprising,” Heimburg said, with a smile. “How would you have the idea to put drunken tadpoles under pressure?”
No Tolerance for Debate
To this day, Heimburg is frustrated by the way biologists react to his ideas, which he calls soliton theory (a soliton is a self-sustaining wave that maintains its shape as it travels). He has faced opposition from the moment he published his theory in 2005 in the Proceedings of the National Academy of Sciences USA, despite that journal’s high regard.
One critic, Catherine Morris, a prominent neurobiologist emeritus at the Ottawa Hospital Research Institute, told me that the whole line of work reeks of superiority from a physicist who thinks he can simply march into a different field and set people straight. She summed this up in a favorite witticism of hers: “It strikes me as this business that physicists do, saying, ‘We can approximate this cow as a single point.’”
To some extent, Morris’s reaction is understandable. It is one thing to say that nerves are mechanical as well as electrical. It is quite another to reject the concept that ion channels play a role in nerve conduction—which Heimburg and Schneider do, in their biggest and most problematic departure from mainstream biology. Never mind that scientists have discovered hundreds of ion channel proteins. Or that the ion flows can be selectively altered with drugs. Or that mutations scientists can create in the proteins change the way neurons fire. “They just blithely ignore vast amounts of biology,” says Morris, who spent 30 years studying ion channel proteins.
Heimburg and Schneider acknowledge that these proteins must serve some function. But they point to experiments, some by Heimburg, showing that ions can flow across artificial membranes even without channel proteins. They attribute this flow to transient holes that appear as the membrane shifts between fluid and liquid-crystal phases, and they think it happens in nerves in the body and brain.
Their skepticism reflects a cultural tendency in physics: a belief that all things should be explainable through thermodynamic principles. Biologists, they say, have neglected these principles as they fixate on proteins. A similar brand of puritanism may have facilitated the eventual dismissal of Tasaki’s theory. He “did not like the term ‘ion channels,’” said former postdoc Iwasa when we spoke in late 2017. This iconoclastic outlook may have guided Tasaki to discover things that others could not have, Iwasa said, “but later on, it may not have helped” him.
Brian Salzberg agrees. He studies nerve physics at the University of Pennsylvania and began his neuroscience career in 1971, crossing paths occasionally with Tasaki. “He was a very clever experimenter, and I have no doubt that he measured real changes” in nerve thickness, Salzberg said earlier this year. “But he misinterpreted them.” Salzberg says nerve fibers temporarily swell as a voltage pulse goes by in part because water molecules flow into the membrane through the same ion channels that let in sodium and then flow back out through the ion channels that let out potassium. If Tasaki had accepted the idea of ion channels, he might have been open to other interpretations of the mechanical wave.
But another powerful factor may have helped push Tasaki out of sight—holding an important lesson for all of science today.
Ideologues
It is intriguing that the thermal energy of a firing nerve may be twice as large as the energy in the electric signal that has dominated neuroscience. The fact that these nonelectric features fell out of favor may stem, in part, from a quirk of history.
Tasaki was a gifted instrument builder who cut his scientific teeth in Tokyo during World War II. Faced with severe equipment shortages, he assembled his own instruments from stray electric components. Years later in the U.S., he used these skills to build exquisite, one-off instruments that measured the heat, or temporary expansion, of nerve cells.
Those devices, and expertise, never found their way to other scientists. Measuring the electric nerve signal was different. Scientists created easily transferable methods, such as inserting a tiny electrode into a cell membrane. As these techniques spread from one lab to another, so did the electrical view of nerve signaling. “There’s a cultural bias,” Parsegian admitted. “People look with a tool that they feel they understand, and they don’t use one that they don’t understand. It could have tilted the thinking.”
Today the technical gaps are starting to disappear. As I checked in with Heimburg between 2011 and 2018, he gradually repeated one old experiment after another, using modern technologies to clarify the surprising things that Tasaki and others first saw decades ago. In 2014 Heimburg redid the drunken-tadpole experiment, using synthetic membranes instead of animals: as he cranked the pressure up to 160 atmospheres, the impacts of anesthetics were reversed—except that this time, Heimburg could link the effect directly to phase changes in the membrane. In 2016 he used microscopy to precisely measure, in a single cell, the mechanical wave that Tasaki and Iwasa first documented in 1979.
Heimburg, now 58, is seeking funding for what could be the most critical experiment of all: measuring the heat as a nerve pulse, or action potential, passes by. Tasaki had measured heat from bundles of fibers, but Heimburg plans to use a microchip that will measure the heat blip of a single neuron. This experiment could address a key criticism of his theory: that a nerve membrane’s brief phase change from liquid to crystal should release, and reabsorb, more heat than Tasaki ever saw. Heimburg contends that the old experiments systematically underestimated the heat; because they measured many neurons, the heat reabsorption after early pulses canceled out the heat releases of later pulses. “The true signal is probably much higher,” he told me in late 2017. If his measurements bear out, they could bolster his claim that the membrane transmits a mechanical wave.
Perhaps most significantly, other scientists are stepping in—outsiders who are not polarized by the old, calcified disputes. Nongjian Tao, a biosensor engineer at Arizona State University, is using lasers to track mechanical pulses in single nerve cells—like Tasaki and Iwasa did, except that Tao reflects his light directly off the nerve rather than a tiny platinum mirror, making the measurement more sensitive. He hopes to monitor hundreds of individual neurons in nerve networks at once, with lasers sensing mechanical waves as they ripple to-and-fro. Such work could answer a key question. “The existence of these [mechanical] effects is not in doubt,” says Simon Laughlin, a neuroscientist at the University of Cambridge. “The question is whether neurons actually use them to do something useful.”
Laughlin does not work on mechanical waves, but as someone who has studied ion channels for 45 years, he imagines that the waves could influence the little protein valves. Recent experiments show that the valves are extremely sensitive to mechanical forces in the membrane. If mechanical waves help to open and close ion channels, that could profoundly change our understanding of the brain because firing neurons mediate all thinking. Ion channels are notoriously noisy and jittery: even tiny thermal vibrations can cause them to pop open or close randomly. Information theorists have struggled for decades to explain how the brain can achieve reliable cognition using such unreliable channels. But mechanical waves could mean the openings and closings are purposeful. “That’s a definite possibility,” Laughlin says.
There are hints that this could be true. Some neurons in the mammalian cortex seem to violate the Hodgkin-Huxley theory. When they fire at high rates, their ion channels open more quickly, as a group, than expected. One explanation is that the channels are responding en masse to a sudden change in the membrane—the arrival of a mechanical wave that opens them more or less in unison, allowing them to fire faster than they otherwise could. The speed might allow them to transmit information at phenomenally quick rates—a possible basis for cognition. In this view, a nerve pulse is both electrical and mechanical.
Heimburg and Schneider occupy a strange place in all of this. They could perhaps one day share a Nobel Prize. Or they could end up nowhere, transfixed by the same insistence that gripped Tasaki for so many decades. The fact that some neuroscientists such as Laughlin and other experts such as Tao are interested in mechanical waves would seem like an important opening for the physicists. But Heimburg was steadfast when we spoke in early 2018. “What many people try to do is somehow rescue the Hodgkin-Huxley model by just combining it with the view that we have,” he said. “But I personally... would not accept any kind of compromise between the two models.”
How Do Nerves Send Signals?
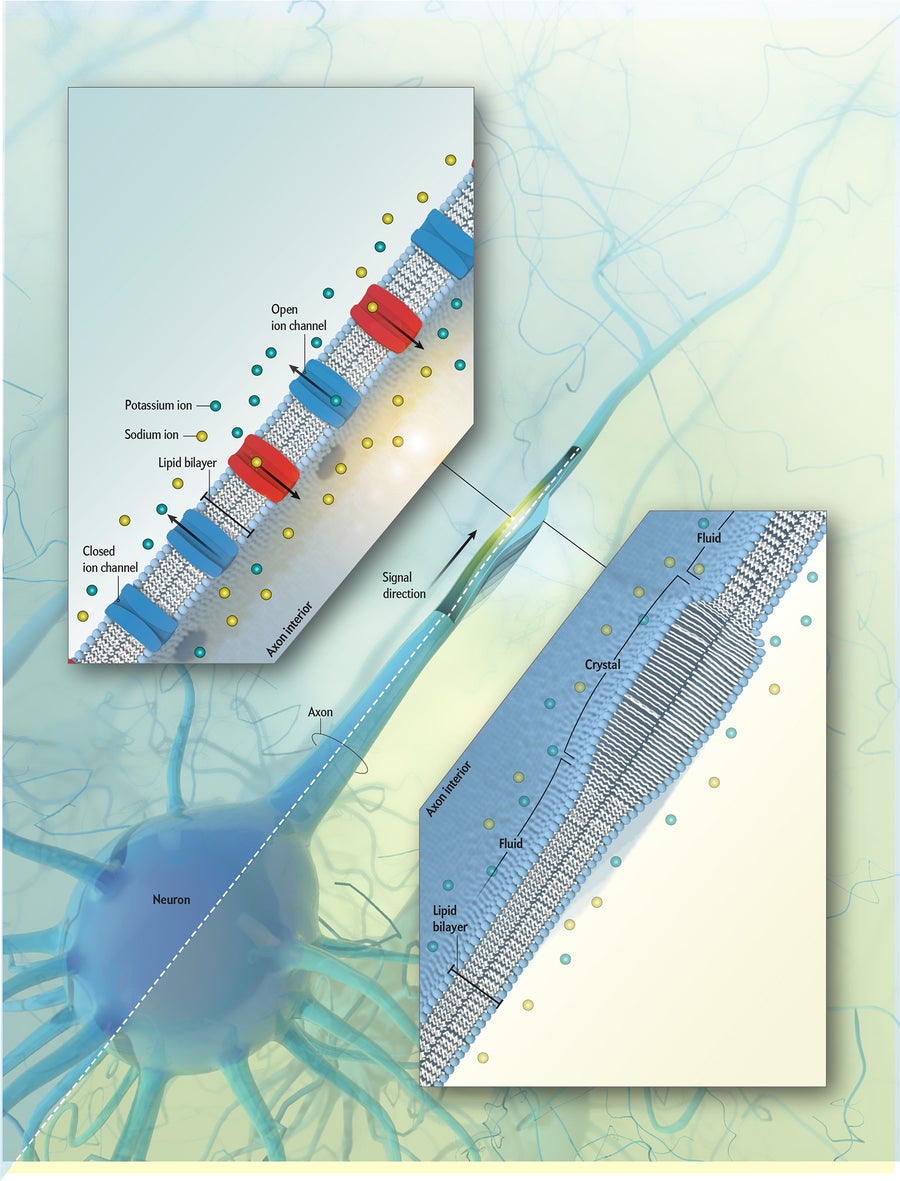
Credit: Falconieri Visuals
For decades scientists have endorsed a standard explanation for how nerve cells (neurons) transmit signals in the body and brain: each message is carried as an electric impulse that travels down a cell’s long axon, jumping to the next neuron. But now a handful of physicists, who have performed exotic tests on the cells in action, say the signal is actually a mechanical pulse that ripples down the axon—akin to a sound wave or seismic wave. Some researchers say any physical pulse is just a side effect of the electric impulse. Settling these contentions could revise explanations of how the brain works.
Prevailing Idea: Electric Pulse
In the conventional view, a nerve signal is transmitted in the membrane that makes up the axon’s outer wall. The membrane is made of lipid molecules. Channels in the lipid layer open momentarily, letting sodium and potassium ions (charged particles) flow through the membrane and then close. As the opening and closing advances down the axon, it creates a traveling voltage pulse.
New View: Mechanical Wave
In the new view, a nerve signal is also transmitted in the axon’s membrane but as a shock wave that travels down the axon. As the wave front advances, it squeezes the lipid molecules, briefly changing them from fluid to liquid crystalline, making them bulge and release heat. As the wave passes, the molecules revert back to fluid form, narrowing and reabsorbing the heat.
