Abstract
Study design:
Experimental study.
Objectives:
To determine the effects of extracorporeal shock wave lithotripsy (ESWL) on the rat spinal cord.
Methods:
Animals were randomly divided into three groups. Groups 1 and 2 consisted of five rats each that underwent ESWL (2000 impulses at 15 kV and 2000 impulses at 18 kV, respectively) and group 3 contained five control rats (no shock wave treatment). ESWL-treated and control rats were compared with regard to light and electron microscopic findings of the adjacent spinal cord.
Results:
Gross neurological outcomes were normal in all groups. Light microscopic examination of group 1 showed extensive extravasation of red blood cells over all the interstitial spaces. Group 2 also had haemorrhagic areas and an irregular organization of axons in the white matter. Transmission electron microscopic examination of group 1 indicated extravasated red blood cells through the endothelium and swollen axoplasm, degenerated mitochondria, destruction of myelin sheaths and a slight increase in the number of lysosomes. Extravasated red blood cells were also seen in group 2. The axoplasmic mitochondria were enlarged, but no sign of mitochondrial degeneration was observed. Lamellar degeneration of myelin sheaths and abundant lysosomes were more predominant in group 2 than in group 1.
Conclusion:
Extracorporeal shock wave lithotripsy caused not only haemorrhage but also damage to neuronal structures except the nucleus. Our findings showed that higher-energy ESWL caused more myelin degeneration in the spinal cord.
Similar content being viewed by others
Introduction
Extracorporeal shock wave lithotripsy (ESWL) has revolutionized the management of urinary stones, and this treatment option is now associated with fewer risks than traditional surgical treatment. The types of shock wave generators include electrohydraulic, electromagnetic and piezoelectric devices. These waves are then focused on the urinary stones.1 Regardless of the source, all shock waves have the capacity to fragment stones. Erosion and shattering result from the application of shock waves followed by stone fragmentation.2 Haemorrhage and oedema, which can be seen in and around the kidney, are the renal side effects of ESWL.1 The extent of the haemorrhage is directly correlated with the kilovoltage used and the number of shock waves administered.3 However, acute extrarenal complications of ESWL can occur. The most common of these are upper gastrointestinal system complications, such as gastric and duodenal erosion;4 rarer extrarenal complications have been described in case reports, with the injury of visceral organs (liver, spleen, pancreas, lungs) being the most frequently noted.5 Ruptures of abdominal aortic aneurysms, ureterovaginal fistulas and ureterocolic fistulas and perforation of the upper ureter have also been reported.5, 6 A study has also described the short-term effects of shock waves on the rat ovary, indicating microscopic haemorrhages and some hypervascularity, but this apparently did not influence ovarian function or gestation.7 Moran et al.8 described disastrous effects of shock waves on chick embryos; this study has served as the basis for the contraindication of ESWL during pregnancy.
The terminal part of the spinal cord and/or roots of the spinal cord in the upper lumbar spinal region can be deleteriously affected by shock wave energy during the ESWL procedure owing to their relationship with the kidneys. ESWL treatment is common but data are limited regarding the complications of this procedure, especially in neural tissue. As we are not aware of any prior studies about the possible effects of ESWL with regard to adjacent neural tissue, we sought to investigate the clinical and histopathological effects of ESWL on the spinal cord.
Materials and methods
This study was approved by the institutional ethical board of our medical school and was performed under institutional guidelines for the care and use of animals in research. Fifteen 1-year-old male Wistar albino rats (250–300 g) were used. The rats were divided into three equal groups. Groups 1 and 2 consisted of five rats each that underwent ESWL (2000 impulses at 15 kV (low energy) and 2000 impulses at 18 kV (high energy), respectively). Group 3 contained five control rats (no shock wave treatment). All groups of rats were anaesthetized by intraperitoneal administration of ketamine (8 mg/100 g; Eczacibasi, Istanbul, Turkey). Rats were positioned prone on the operating table. The thoracolumbar area of each rat was shaved, and the operative field was prepared in a sterile manner using a povidine–iodine solution. A dorsal midline skin incision was made with a number 15 blade and continued down to the spinous process. We put a radiopaque vascular clip in the T12-L1 interspinosus space just beneath the last thoracic vertebra's processus spinosus. After haemostasis, the wound was closed using 3-0 silk suture material, and the animals were allowed to recover. No prophylactic antibiotics were used. Subsequently, Group 1 and 2 rats were re-anaesthetized, and a single shock wave treatment was applied to the spinal cord. A shock wave treatment was applied to the spinal cord using an electrohydraulic machine (Stonelithotripter P3500-05030-52; PCK, Ankara, Turkey). The shock waves were directed at the posterior aspect of the T12–L1 spine. The location of the spinal canal was determined by following the radiopaque vascular clip under C-arm imaging. The first group of rats received 2000 impulses of shock waves at 15 kV, and the second group received 2000 impulses of shock waves at 18 kV. We chose this shock wave energy because it was close to that used clinically in humans. Immediately after ESWL treatment, the treated dorsal spinal skin was inspected for local punctuate haemorrhages. The control rats received no shock wave treatment. The rats were returned to their cages after ESWL treatment. A surgical wound and neurological examination was conducted every day for 1 week. Any superficial wound infections were treated with povidine–iodine without using antibiotics. All animal motor performance examinations were evaluated using a modified Tarlov score (from 0 for paraplegic to 5 for normal rats).9 All animals were killed by ketamine overdose at 1 week after the procedure. The thoracolumbar spine was removed en bloc. The injured 1-cm spinal cord segment, at the level of T12–L1, was excised after laminectomy, opening the dura and cutting the roots under a surgical microscope. All the sections were examined by histologists (TZ, BC) who were blinded to the groups.
Light microscopy
Five spinal cord segments from the control group and five segments from each ESWL group were fixed in 10% neutral formalin (pH 7.4) for at least 24 h at room temperature. Then, tissue specimens were processed for routine histology as follows: tissues were dehydrated in an ascending series of ethanol solutions, cleared in xylene and perfused with liquid paraffin before being embedded in paraffin wax. Serial sections (5 μm) were cut. The sections were stained with haematoxylin and eosin for routine histological examination and with crystal violet to analyse perikaryonic structures. Paraffin sections were examined under a bright field microscope (Eclipse E600; Nikon, Tokyo, Japan) and images were captured using a Nikon Coolpix 5000 digital camera attachment.
Transmission electron microscopy
Tissue pieces of white matter and grey matter from all animals in the three groups were fixed in 2% glutaraldehyde in phosphate buffer (pH 7.4) for 4 h. Specimens were then post-fixed in 1% osmium tetroxide for 2 h. Tissue blocks were stained with 0.5% uranyl acetate, dehydrated and embedded in propylene/araldite embedding media (Electron Microscopy Sciences, Hartfield, PA, USA). Each block was cut into serial semi-thin sections (700 nm), which were stained with toluidine blue/azur II. Appropriate portions of the sections were then cut into 7-nm-thick ultrathin sections and stained with uranyl acetate and lead citrate. Sections were observed under an LEO 906E transmission electron microscope (LEO Elektronmikroskopie, GmbH, Oberkochen, Germany).
Results
All animals survived and their postoperative course was uneventful. All rats had a modified Tarlov score determined as 5 during examination every day for a week post-procedure.
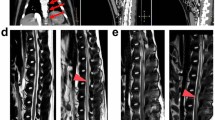
Light microscopic examination of the control group sections demonstrated normal structure of white matter and grey matter elements (Figures 1a and b). Group 1 exhibited extensive extravasation of red blood cells over the entire interstitial space, under the pia mater and in the central canal (Figure 1d). Other than that, the white matter and grey matter appeared normal (Figures 1c and d). Group 2 had normal grey matter appearance (Figure 1e) as well as haemorrhagic areas (Figure 2a). In addition, an irregular organization of the axons was observed in the white matter, and myelin coats appeared to have varying diameters (Figure 1f).
All groups revealed normal perikaryonic appearance (a, c, e). The control group showed normal white matter structure and organization (b). Both of the extracorporeal shock wave lithotripsy (ESWL) groups had haemorrhagic areas, but it was more evident in group 1, with haemorrhage into the central canal (d, arrowhead). Group 2 demonstrated irregular organization of axons as well as myelin sheaths of different sizes (f). Haematoxylin and eosin staining. Scale bar=0.05 mm (a, c, e); 0.1 mm (b, d, f).
Extracorporeal shock wave lithotripsy (ESWL) groups showed extensive extravasation of red blood cells (a). Red blood cells were also observed in the stroma by transmission electron microscopy (b, d, arrowheads) but capillaries appeared intact (c, e, en: endothelial cell nucleus, asterisks: red blood cell in the capillary lumen). (a) Haematoxylin and eosin staining, Scale bar=0.1 mm. (b, d) × 1293; (c, e) × 2784.
Transmission electron microscopic examination of group 1 showed extravasated red blood cells (Figure 2b), although the endothelium and basal lamina appeared intact (Figure 2c). Degenerated axoplasms with swollen, degenerated mitochondria of various sizes were observed (Figure 3e). Destruction of myelin sheaths was evident, with a lamellar appearance and irregular thickness (Figure 3b). Nuclear and perikaryonic structures appeared normal, with the exception of a slight increase in the number of lysosomes (Figure 3k). Extravasated red blood cells were also seen in group 2 (Figure 2d, arrowheads), but the blood vessels showed no sign of erosion (Figure 2e). Lamellar degeneration of the myelin sheaths was more obvious (Figure 3c). The axoplasmic mitochondria were enlarged, but no sign of mitochondrial degeneration was observed. However, pieces of damaged myelin were occasionally seen inside the axoplasm (Figure 3f). Nuclear and perinuclear organelles were normal; however, lysosomes were more abundant than in group 1 (Figure 3l).
The control group showed normal myelin, axoplasmal structures (a, d, m: myelin sheath, a: axoplasm, mit: mitochondria), perikaryon and perikaryonic organelles, (g, j, n: nucleus, rER: rough endoplasmic reticulum, mit: mitochondria). Group 1 had degenerated myelin (b, asterisk), swollen and degenerated mitochondria in the axoplasm (e, thick arrow), normal cytoplasm and organelles (h) and an increase in the number of lysosomes (k, arrowheads). Group 2 had lamellar degeneration of myelin (c, f, asterisks) and enlarged axoplasmic mitochondria (f, thick arrows). The perikaryon and organelles appeared normal (i) while the number of lysosomes increased more than in group 1 (l, arrowheads). n: nucleus. (a, b) × 3597; (c) × 1670; (d, g, i–l) × 6000; (e) × 7750; (h) × 4646.
A summary of histopathological findings can be found in Table 1.
Discussion
Extracorporeal shock wave lithotripsy, compared to surgical treatment, is typically a painless, bloodless and a relatively low-risk method. It is now usually the first treatment choice for both renal and ureteral calculi. Renal biopsies taken 1 week after ESWL showed oedema and extravasation of urine and blood into the interstitial spaces, blocking of cortical tubules by haemorrhagic streaks and widespread dilation of the veins, with signs of endothelial destruction and partial organization of thrombi under light microscopy.10 Experimental studies in a rat model revealed that after 1000 shock waves at 18 kV, only 5 of 20 treated kidneys appeared to be normal or minimally affected, whereas 15 showed gross evidence of marked vascular injury.11
To our knowledge, no detailed experimental study had examined the effects of ESWL treatment on the spinal cord. Newman et al.14 published a laboratory study on the adverse effects of ESWL treatment on neurological tissues. They observed hind limb weakness after shock wave application to the dog ureter. They did not quantify the ESWL dosage applied. The weakness resolved within 48 h and the spinal cord postmortem examination was normal. They did not describe how they examined the spinal cord. Nonetheless, this finding may be due to injury to the lumbar plexus because of its close proximity to the ureter. They also investigated the effect of ESWL on the canine spinal cord; 2000, 4000 and 6000 shocks were directed at the posterior aspect of the L3–L4 or L4–L5 interspace in three dogs. They noticed no clinical effect, but the published report was not very detailed.14
Neurological complications of ESWL treatment in syringomyelia patients have been reported. Lorenzo et al.12 described a case of post-traumatic thoracic syringomyelia that presented with acute, rapidly progressive flaccid paraplegia and urinary retention following ESWL treatment for ureteral stones. They proposed that the water-like fluid of the syrinx cavity was a better conductor than the spinal cord for transferring shock waves, which led to the serious and irreversible changes to the nervous system surrounding the cavity. In addition, Tosi and Terini13 reported a case of acute urinary retention in a patient with post-traumatic syringomyelia following ESWL administration. The patient's magnetic resonance imaging study showed enlargement of the cystic cavity after ESWL treatment.
Although no gross neurological changes were seen in our study, significant differences were observed within groups on histopathological examination. ESWL caused not only haemorrhaging but also damage to neuronal structures except the nucleus. Transmission electron microscopic examination of group 1 showed extravasated red blood cells through the endothelium, swollen axoplasms, degenerated mitochondria, destruction of myelin sheaths and a slight increase in the number of lysosomes. The latter changes reflect the cells’ efforts to clear the damaged organelles. Extravasated red blood cells were also seen in group 2, which also exhibited lamellar degeneration of the myelin sheaths. The axoplasmic mitochondria were enlarged, but no sign of mitochondrial degeneration was observed. Lysosomes were more abundant than in group 1. If more significant mitochondrial degeneration had occurred, normal cell function could have been disrupted. The extent of myelin degeneration and the increase in lysosome number was more severe in rats that were exposed to higher kilovoltage. Myelin is essential for normal nerve physiology, and myelin degeneration is an important contributor to functional loss in many central nervous system disorders, including trauma, multiple sclerosis and stroke.15 The increase in the lysosome number might be responsible for the removal of the damaged tissues.
Extracorporeal shock wave lithotripsy treatment appears safe when treating ureteral or renal stones lying near the vertebral column, only if gross neurological results are considered. However, our preliminary data showed that EWSL caused damage to the spinal cord, and to our knowledge this is the first study to demonstrate that ESWL was responsible for spinal cord effects histopathologically. The histopathological changes apparently did not cause clinically obvious deficits in the rats. However, we only evaluated neurological and histopathological outcomes 1 week after a single-dose ESWL application. Higher and fractioned doses may cause significant damage to the spinal cord. Further animal studies are needed to examine longer-term outcomes, electrophysiological and biochemical effects and whether the histological results are permanent. In addition, ESWL can serve as an experimental model of rat spinal cord injury due to the neuronal degeneration observed.
References
Lingeman JE, Matlaga BR, Evan AP . Surgical management of upper urinary tract calculi. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (eds). Campbell–Walsh Urology, 9th edn. WB Saunders Elsevier: Philadelphia, PA, 2007, pp 1431–1506.
Stoller ML . Extracorporeal shock wave lithotripsy. In: Tanagho EA, McAninch JW (eds). Smith's General Urology, 14th edn. Appleton & Lange: Stamford, CT, 1995, pp 305–313.
Newman R, Hackett R, Senior D, Brock K, Feldman J, Sosnowski J et al. Pathologic effects of ESWL on canine renal tissue. Urology 1987; 29: 194–200.
Karawi MA, Mohamed AR, el-Etaibi KE, Abomelha MS, Seed RF . Extracorporeal shockwave lithotripsy (ESWL)-induced erosions in upper gastrointestinal tract. Prospective study in 40 patients. Urology 1987; 30: 224–227.
Finter F, Rinnab L, Simon J, Volkmer B, Hautmann R, Kuefer R . Ureteral stricture after extracorporeal shock wave lithotripsy: case report and overview of the spectrum of rare side effects of modern ESWL treatment. Urologe A 2007; 46: 769–772.
Turgut M, Can C, Yenilmez A, Akcar N . Perforation of the upper ureter: a rare complication of extracorporeal shock wave lithotripsy. Urol Res 2007; 35: 215–218.
McCullough DL, Yeaman LD, Bo WJ . Effects of shock waves on the rat ovary. J Urol 1989; 141: 666–669.
Moran ME, Sandock D, Bottaccini MR . Chick embryo model to study effects of high energy shock waves on rapidly proliferating tissues. J Endourol 1990; 4: 315.
Ridet JL, Pencalet P, Belcram M . Effects of spinal cord X-irradiation on the recovery of paraplegic rats. Exp Neurol 2000; 161: 1–14.
Rigatti P, Colombo R, Centemero A, Francesca F, Di Girolamo V, Montorsi F et al. Histological and ultrastructural evaluation of extracorporeal shock wave lithotripsy-induced acute renal lesions: preliminary report. Eur Urol 1989; 16: 207–211.
Weber C, Moran ME, Braun EJ, Drach GW . Injury of rat renal vessels following extracorporeal shock wave treatment. J Urol 1992; 147: 476–481.
Lorenzo ND, Maleci A, Williams BM . Severe exacerbation of post traumatic syringomyelia after lithotripsy. Paraplegia 1994; 32: 694–696.
Tosi L, Terini G . Lithotripsy in SCI patients. Letter. Paraplegia 1995; 33: 364.
Newman RC, Hackett RL, Brock KA . Effect of ESWL on canine spinal cord. Urology 1987; 29: 116.
Totoiu MO, Keirstead HS . Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 2005; 486: 373–383.
Acknowledgements
This study was supported by a grant from the Turkish Neurosurgical Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, see: http://www.textcheck.com/cgi-bin/certificate.cgi?id=tMniUx.
Rights and permissions
About this article
Cite this article
Karatas, A., Dosoglu, M., Zeyrek, T. et al. The effect of extracorporeal shock wave lithotripsy on the rat spinal cord. Spinal Cord 46, 627–632 (2008). https://doi.org/10.1038/sc.2008.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.31