Abstract
Background:
Paraplegia complicating surgical thoracoabdominal aneurysm (TAA) repair remains an unpredictable and poorly understood phenomenon. The ability to identify patients at increased risk of delayed paraplegia before the process becomes irreversible could allow early interventions to attenuate this risk.
Methods:
In a rabbit model of infra-renal spinal cord ischemia, serial T2 weighted (T2W) magnetic resonance (MR) imaging was performed 2- and 8 h after the ischemic insult with changes correlated with clinical outcome. Using the axial T2W images, signal intensity measurements of the lateral horns of the spinal cord were acquired, both above (that is, thoracolumbar cord) and below (that is, lumbar cord) the renal arteries. This ratio (lumbar/thoracolumbar cord signal intensity) was evaluated and compared between groups.
Results:
No changes were seen in the signal intensity of rabbits that remained neurologically intact. Rabbits with delayed paralysis showed a significant (P<0.01) decrease in signal intensity ratio at 2 h (1.13±0.03), while a significant (P<0.01) increase was noted in those rabbits with immediate persistent paralysis (1.43±0.04). There was a significant (P<0.01) increase in the signal intensity ratios at 2 h in the delayed paralysis group (1.55±0.14), with a further significant (P<0.01) increase at 8 h in the immediate persistent paralysis group (1.76±0.07).
Conclusions:
Findings on MR imaging can differentiate clinical outcomes in this experimental model of spinal cord ischemia. While further studies are required, MR could be useful in predicting which patients are at risk for delayed paraplegia after TAA repair.
Similar content being viewed by others
Introduction
Paraplegia continues to be a frequent and catastrophic complication of surgical thoracoabdominal aneurysm (TAA) repair, with an incidence of up to 33% reported.1 Mechanisms involved are potentially multiple; however, spinal cord ischemia at the time of surgery is believed to be the major factor. Not all cases of paraplegia are evident immediately postoperatively; a subset of patients with no evidence of neurologic deficit early after surgery may subsequently progress to paraplegia. Although the mechanisms of delayed-onset paraplegia remain obscure, growing evidence supports neuronal-cell apoptosis as central in the pathogenesis of this phenomenon.2
Numerous therapeutic approaches have been studied in attempts at preventing paraplegia after TAA repair.3, 4 Despite development of potentially useful strategies that help attenuating the effects of spinal cord ischemia, paraplegia continues to be a significant clinical problem.
An imaging modality that could identify patients at risk of paraplegia, particularly delayed paraplegia, after TAA repair would allow early and intensive therapeutic intervention in this sub-population. The overall cost:benefit ratio of postoperative care could, therefore, be improved by permitting therapy directed to patients identified to be at increased risk.
Magnetic resonance (MR) imaging is known to provide high-resolution information about the brain, and to be sensitive to perturbations in pathophysiology. In particular, T2 weighted (T2W) imaging can detect regions of abnormality where increased water content due to injury—ischemic or otherwise—is identified as a region of increased signal intensity (that is, bright). Although the ability of MR imaging to document changes in the spinal cord as a result of ischemia has been reported,5 development of this technique using reproducible experimental models could lead to the ability to differentiate and predict different clinical outcomes.
We therefore performed a preliminary study of serial MR imaging to detect changes in signal intensity within the spinal cord in a rabbit model of spinal cord ischemia at different times after the ischemic insult, and correlated these results with clinical outcome.
Methods
Animal model of spinal cord ischemia
All procedures were approved by the Institutional Animal Care and Use Committee, and animal care complied with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington: National Academy Press, 1996.
Male New Zealand white rabbits (n=16, weight 3.0–3.5 kg) were used. Spinal cord ischemia was induced by a method validated previously.6 Animals were anesthetized with ketamine (40 mg/kg intramuscular, Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (10 mg/kg intramuscular, Bayer Corporation, Shawnee Mission, KS, USA). Under sterile conditions, a 4-cm left flank incision was used to access the abdominal aorta extraperitoneally. A snare device was positioned around the aorta, just distal to the origin of the left renal artery, and tunneled through the posterior musculature to the skin, inside a 12-Fr polyurethane tube. As a small Rommel tourniquet secured externally, this device allows later access for aortic occlusion. The wound was closed in layers around the tubing with 3-0 silk sutures, and the animals recovered.
MR imaging protocol
Unoperated rabbits (n=4) underwent MR imaging to optimize the imaging sequences and document MR characteristics of the normal rabbit spinal cord. These animals were anesthetized with ketamine and xylazine, as described above, and placed supine in a clinical 1.5 Tesla MRI system (Signa, General Electric Medical Systems, Milwaukee, WI, USA), using a conventional phased-array volume coil as described previously.7, 8 Fast gradient-echo coronal images were used to localize the spinal cord; and sagittal and axial T2W fast spin echo images of the spinal cord were acquired from the diaphragm to the sacrum. Imaging sequences used were (1) Sagittal T2W images with TR/TE: 4000/102 msec, FOV 20 × 15 cm, matrix 256 × 256 (in-plane resolution: 0.8 × 0.6 mm), number of signal averages 3, echo train length 12, slice thickness 2 mm; and (2) axial T2W images with TR/TE: 5000/102 msec, FOV 9 × 9 cm, matrix 256 × 256 (in-plane resolution: 0.35 × 0.35 mm), number of signal averages 2, echo train length 8, slice thickness 3 mm. The entire imaging protocol was completed in less than 10 min.
Signal intensity analysis
Using the axial T2W images, signal intensity measurements of the lateral horns (that is, two measurements per axial MR image) of the spinal cord were made from three consecutive MR images both above (that is, thoracolumbar cord) and below the renal arteries (that is, lumbar cord), using a region of interest analysis tool. This image sampling allowed analysis of the signal intensity characteristics of the cord above and below the site where ischemia was induced. The mean of six signal intensity measurements from the spinal cord above the renal arteries was used as denominator for the ratio with six signal intensity measurements taken from the lumbar cord.
Study design
Infrarenal aortic occlusion for 18 min was performed 48 h after the surgery. This procedure was carried out with animals awake, to allow assessment of motor response of the lower extremities. Adequacy of tightening of the circumaortic snare was confirmed in all animals by development of paralysis during the procedure. Postoperatively, neurologic status was scored using the modified Tarlov criteria. A score of 0–4 was assigned to each animal, as follows: 0, no voluntary hindlimb function; 1, movement of joints perceptible; 2, active movement but unable to stand; 3, able to stand but unable to walk; and 4, complete recovery of hindlimb function. MR imaging was performed in all rabbits at 2- and 8 h after spinal cord ischemia. Each animal was recovered after each MR scan and re-assessed neurologically. All paralyzed animals were euthanized after the final MR scan; the remaining animals were serially evaluated during 24 h after aortic occlusion to determine the development-delayed paralysis. Euthanasia was accomplished by intravenous injection of ‘Sleepaway’ (5 ml, Fort Dodge Animal Health).
Statistics
All values are expressed as mean±s.e.m. Statistical analyses were performed using paired and unpaired Student's t-tests for comparing values within a same animal, and between different animals, respectively. A P-value of <0.05 was used to indicate statistical significance.
Results
Spinal cord ischemia and paralysis
After spinal cord ischemia was induced with the aortic snare, four rabbits recovered full neurological function and eight remained paralyzed. After the 2-h MR imaging protocol, no new neurological findings were noted; however, one of the paralyzed rabbits died after completing MR scan. At the 8-h MR imaging protocol, two of the rabbits that had recovered full neurological function were paralyzed. The two remaining rabbits with full neurological function remained normal until euthanasia, 24 h after spinal cord ischemia. Therefore, with this experimental model, two rabbits remained normal throughout the study, two experienced delayed paralysis and eight had immediate persistent paralysis.
T2W MR imaging
The mean signal intensity ratio of the lumbar spinal cord segments of normal rabbits, used in the validation pre-study, was 1.29±0.03 (thus the signal intensity from the lumbar spinal cord segments is slightly higher than that of the thoracolumbar region above the renal arteries).
The rabbits that remained normal throughout the study period showed no significant change in signal intensity ratio over the controls at 2 h (1.29±0.07). The rabbits with delayed paralysis showed no increase, but a significant (P<0.01) decrease in signal intensity ratio at 2 h (1.13±0.03) over the controls. However, already a significant (P<0.01) increase was noted in the signal intensity ratio in those rabbits with immediate persistent paralysis (1.43±0.04).
Comparing the signal intensity ratios at 2- and 8 h in the same animals allowed direct comparison of the same spinal cord segments over time (Figures 1, 2 and 3). In those rabbits that remained normal throughout the study period, there was no significant change at 8 h (1.15±0.03) in signal intensity ratios (Figure 4a). There was a significant (P<0.01) increase in the signal intensity ratios at 8 h in the delayed paralysis group (1.55±0.14; Figure 4b). There was a further significant (P<0.01) increase in the signal intensity ratios at 8 h in the immediate persistent paralysis group (1.76±0.07; Figure 4c).
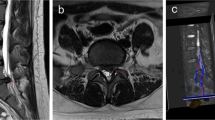
T2W MR images from a normal rabbit. These images show the spinal cord tissue on both (a) sagittal and (b) axial projections. (c) Magnification of the axial MR image, showing the ability to define the spinal cord tissue and identifying the regions from where the signal intensity measurements were made (white arrows). T2W, T2 weighted; MR, magnetic resonance.
Montage of magnified axial T2W MR images of the spinal cord from rabbits in different groups at different time points. MR images at (a) 2 h and (b) 8 h from a rabbit that remained neurologically normal throughout the study period despite the induction of spinal cord ischemia. MR images from the (c) 2- and (d) 8-h time points from a rabbit in the delayed paralysis group. MR images at the (e) 2- and (f) 8-h time points as described for a rabbit that experienced immediate and persistent paralysis after spinal cord ischemia. T2W, T2 weighted; MR, magnetic resonance.
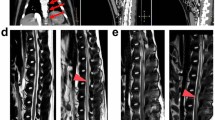
Magnification of the 8-h MR scans from Figure 2, demonstrating the signal changes within the spinal cord. The signal intensity of the lateral horns (white arrows) of the normal (unparalyzed) rabbit (a) appears homogenous with that of the rest of the cord. However, the signal intensity of the lateral horns (white arrows) is increased in the rabbit with delayed paralysis (b) and higher again in the same region in the rabbit with immediate paralysis (c). MR, magnetic resonance.
Discussion
The ability to distinguish different clinical outcomes in this rabbit model of spinal cord ischemia using T2W MR imaging is a preliminary but potentially important finding. We demonstrated that with serial imaging, no significant changes occur in signal intensity in the spinal cord of rabbits that remain normal neurologically, despite the 18-min spinal cord ischemia. In addition, the significant step-wise increase in signal intensity at both 2- and 8 h in the immediate persistent paralysis group confirms the ability to identify early changes in the spinal cord with T2W MR imaging. Of interest and potential importance is that at 2 h a decrease was noted in the signal intensity of the spinal cords of those rabbits which were at that time neurologically normal, but later became paralyzed. Furthermore, an increase occurred in the signal intensity at the time of paralysis.
In the rabbits that remained normal throughout the study period, it is likely no reversible or irreversible injury to the spinal cord occurred, and thus the segments maintained their signal intensity throughout. Those animals paralyzed immediately and persistently may have necrosis of motor neurons within the spinal cord, a phenomenon associated with induction of inflammatory processes and edema.9 The observed increase in signal intensity seen in the spinal cords of this group over time may reflect this process. The pathophysiology underlying the delayed paralysis group may be different. Studies have suggested that in this group there may be an induction of processes that lead to neuronal apoptosis, which does not induce inflammation or edema.9 The interesting finding of a reduction in signal intensity at 2 h in the group with delayed paralysis warrants further evaluation as a potential marker of this outcome.
Limitations
Our ability to draw conclusions about mechanisms involved in the different groups is limited by the lack of histopathology in this study. Furthermore, this was a preliminary study and as such a small number of animals was only studied. However, the feasibility of serial MR imaging to identify changes in the spinal cord of rabbits subjected to cord ischemia, and its correlation with neurologic outcome are important findings, which may lead to more resourceful application of MR both clinically and as a research tool.
This work forms the premise for further studies to elucidate the relation between MR findings and the underlying pathologic processes, leading to a clinical evaluation of MRI in such patients. Given the present lack of means to effectively identify patients ‘at risk,’ the potential of MR imaging is promising as a noninvasive diagnostic modality in the management of spinal cord ischemia after TAA repair.
Conclusion
In this rabbit model of spinal cord ischemia, T2W MR imaging signal intensity characteristics are associated with paraplegic outcomes. This imaging modality may be beneficial in detecting and potentially treating patients who develop delayed paraplegia following TAA repair.
References
Panneton JM, Hollier LH . Dissecting descending thoracic and thoracoabdominal aortic aneurysms: part II. Ann Vasc Surg 1995; 9: 596–605.
Sakurai M, Hayashi T, Abe K, Sadahiro M, Tabayashi K . Delayed selective motor neuron death and fas antigen induction after spinal cord ischemia in rabbits. Brain Res 1998; 797: 23–28.
Fan T, Wang CC, Wang FM, Cheng F, Qiao H, Liu SL et al. Experimental study of the protection of ischemic preconditioning to spinal cord ischemia. Surg Neurol 1999; 52: 299–305.
Hamilton INJ, Hollier LH . Adjunctive therapy for spinal cord protection during thoracoabdominal aortic aneurysm repair. Semin Thorac Cardiovasc Surg 1998; 10: 35–39.
Fortuna A, Ferrante L, Acqui M, Trillo G . Spinal cord ischemia diagnosed by MRI. Case report and review of the literature. J Neuroradiol 1995; 22: 115–122.
Naslund TC, Hollier LH, Money SR, Facundus EC, Skenderis II BW . Protecting the ischemic spinal cord during aortic clamping. The influence of anesthetics and hypothermia. Ann Surg 1992; 215: 409–415; discussion 415–416.
Helft G, Worthley SG, Fuster V, Fayad ZA, Zaman AG, Corti R et al. Progression and regression of atherosclerotic lesions: monitoring with serial noninvasive magnetic resonance imaging. Circulation 2002; 105: 993–998.
Worthley SG, Helft G, Fuster V, Zaman AG, Fayad ZA, Fallon JT et al. Serial in vivo MRI documents arterial remodeling in experimental atherosclerosis. Circulation 2000; 101: 586–589.
Hayashi T, Sakurai M, Abe K, Sadahiro M, Tabayashi K, Itoyama Y . Apoptosis of motor neurons with induction of caspases in the spinal cord after ischemia. Stroke 1998; 29: 1007–1012; discussion 1013.
Acknowledgements
This work was supported by grants from the National Heart Foundation of Australia (SA Branch) (SGW) and the French Federation of Cardiology (GH). The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Worthley, S., Reis, E., Helft, G. et al. Serial magnetic resonance imaging correlates with neurological outcome in an experimental model of spinal cord ischemia. Spinal Cord 46, 222–227 (2008). https://doi.org/10.1038/sj.sc.3102108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102108