Abstract
Background:
A number of techniques are being investigated to accomplish bladder control recovery in paralyzed patients using the neurostimulation, but currently, all techniques are based on the dorsal implantation of the electrodes using a laminectomy.
Methods:
On 27 April 2006 we performed a laparoscopic implantation of a Finetech–Brindley bladder controller on the endopelvic sacral roots in a Th8 completely paralyzed woman who had previously undergone the removal of a Brindley controller due to an arachnoiditis after extrathecal implantation with intradural sacral deafferentation.
Results:
We required about 3.5 h for the entire surgical procedure; no complications occurred and the patients went home on 5th postoperative day. The patient is now able to void empty her bladder and her rectum using the controller without further need for self-catheterisation.
Conclusions:
The presented new technique of laparoscopic implantation of electrodes on the endopelvic portion of the sacral nerve roots is an option to be considered in all paralyzed patients with further wish for electrical induced miction/defecation after previous deafferentation.
Similar content being viewed by others
Introduction
The consequences of spinal cord injury to the urinary bladder function are severe and play an important role in the health and well-being of the patient for life. A number of complications, such as cystitis, incontinence, vesicoureteral reflux, hydronephrosis and renal failure are often found in conjunction with spinal cord injury.1 The main factors contributing to these complications are the presence of residual urine and the occurrence of sustained high intravesical pressures. Thus, the most important aim in the therapy of a neurogenous bladder is the preservation of kidney function. For this reason, cystitis is mostly treated with antibiotics, and attempts are made to lower the high pressure in the bladder and to suppress reflex contractions during voiding. Anticholinergic medication or Botulinum toxin installation to the detrusor can be used, but the intermittent voiding of the bladder with a catheter still remains the safest method to protect the kidneys. Some patients feel that self-catheterisation is incompatible with a normal social/professional life. In a study of 1114 patients with a neurogenic bladder, approximately 8% were still using the self-catheterisation method after 5 years, and only 5% after 10 years. In the case of failure of the drug, destructive surgical treatments such as surgical augmentation of the bladder are advocated,2 but a less aggressive alternative option is the use of an implantable neural prothesis for electrical bladder stimulation.
Methods
GH is a 50-year-old woman, completely paralyzed at Th8 by an accident in October 1972. Until 2002, she had a reflectoric bladder and could void her bladder by applying external abdominal pressure. After many years of consideration, she then opted for an extrathecal implantation of a Finetech–Brindley bladder controller on the anterior sacral roots with an intradural sacral deafferentation. The operation was performed in July 2002. Between August and September 2002, revisional operations were performed due to healing disturbances in the flank incision area. It was recommended to the patient that the neurostimulator be removed, but she was unwilling to comply as she was very happy with the functional results. The neuroimplant was finally removed in June 2003 due to arachnoiditis with staphylococcal sepsis caused by an infection of the implanted stimulator and electrodes. The implant as well as the electrode were lying uncovered in the open wound. The patient requested another implantation, but, due to the fact that during the first procedure a combined intra- and extrathecal approach had been used and the patient had developed arachnoiditis and persistent decubitus ulcers in the back and sacral areas, neither an intra- nor an extrathecal reimplantation was possible. Consequently, the patient has been suffering from recurrent cystitis and pyelonefritis that has become more and more difficult to treat with antibiotics and she developed an absolute aversion to self-catheterisation. During the last 2 years, she has spent most of her time being admitted to hospitals for decubitus treatment. She also underwent multiple flap transplants during those admissions. Psychologically, she was ready to give up and put her last hopes on a new implantation of a bladder controller to protect her kidneys and her life. We decided to restore an electrical-induced miction by using functional electrical stimulation (FES) of the sacral nerve roots in their endopelvic portion by implantation of neural electrodes on laparoscopic transperitoneal way.
At the time of the consultation, the patient was very depressed but had a fervent desire to be helped. On clinical examination, no active focus of infection could be detected.
The patient stopped her medication 2 weeks before the procedure and was given an intravenous antibiotic prophylaxis with ceftriaxon starting the day before the procedure, which was continued for the first 3 postoperative days.
During the surgical procedure, a microtip rectal probe and a 8F dual-sensor microtip transurethral catheter with a filling channel were used for intraoperative urodynamic testing, while all other skeletal muscle responses were visually observed. For the laparoscopy, a 10 mm trocar introduced into the umbilicus was used for the endoscope and three further 5 mm trocars were placed in the lower abdomen for the different laparoscopic instruments.
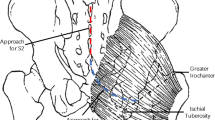
The surgical procedure began with the mobilisation of the rectosigmoid from the sacral bone and full exposure of the endopelvic part of sacral roots S2 to S4 on both sides according to our technique as described previously.3 The contractability of the sphincters and of the detrusor were directly assessed using the laparoscopic neuro-navigation (LANN) technique on the different sacral roots.4
From the surgical report of the previous implantation, we gained the knowledge that the best rectum and bladder contraction had been obtained by stimulation of the third sacral nerve roots on both sides. Using the LANN technique, we were able to confirm it. Because S3 and S4 on both sides, however, were anatomically and extremely close to each other, we decided intraoperatively to implant just one double electrode for simultaneous bilateral stimulation of S3 and S4 and a second double electrode for the elective stimulation of S2 on both sides. Both the double electrodes (extradural electrode forked – Finetech Medical System) were introduced through the 10 mm umbilical trocar and placed on the sacral roots immediately after their emergence out of the sacral foraminae. They were fixed using the silicon rubber of the electrodes. To avoid dislocation of the electrodes, the cables were sutured to the sacral periost with non-resorbable sutures (Figure 1). All the cables were tunnelled retroperitoneally on the left side between the internal iliac artery and the pelvic wall to avoid direct contact with the ureter, the sacral plexus and the obturatoric nerve. They were connected to a new receiver block subcutaneously in the left lower abdomen.
Results
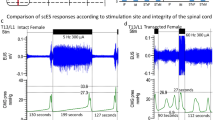
MP performed the procedure on 27 April 2006 in the Department of Gynecology and Obstetrics at St Elisabeth Hospital, Cologne, Germany. The entire procedure took 205 min; the blood loss was estimated at less than 50 ml and no complications occurred. The stimulation of the bladder was started on the third postoperative day, and spontaneous micturition was immediately obtained with residual volumes being less than 50 ml. The patient went home on the 5th postoperative day in good physical form and a positive frame of mind. On 11 May 2006, the patient was seen for a follow-up consultation and the Brindley controller was set up. All the wounds had healed well. Urodynamic testing was then carried out. Bilateral stimulation of S2 resulted in equal contractions of the gluteal muscles. Bilateral stimulation of S3 with a voltage of 40, wavelength of 350 μs and frequency of 25 s resulted in an immediate detrusor contraction up to 40 cm H2O and an intrarectal pressure of 20 cm H20. In BIRST-regime, a typical low resistance ‘post stimulus voiding’ took place. At the 10-week-postoperative follow-up, urodynamic testing again showed a complete voiding of the bladder (Figure 2). The patient is also able to void her rectum by using the neurostimulator. No infections occurred and there has been no recurrence of sacral decubitus ulcers. Nine months after the procedure, the device is working optimally allowing complete emptying of the bladder.
Discussion
A complete biological cure for spinal cord injury is unlikely to be developed in the near future5, and therefore, electrical devices are still required to restore control of the lower urinary and gastrointestinal tracts. A number of techniques based on different kinds of stimulation are being investigated to accomplish this, but until now, no clinical device can be said to have solved the bladder control problem.6 The Finetech–Brindley bladder controller has a number of drawbacks,7 but is at present considered the only clinically available implantable system for bladder control. The bladder is activated by stimulation of the ventral sacral nerve roots using intrathecal hook electrodes implanted on either side of the nerve roots by a dorsal approach, and is combined systematically with a deafferentation of the bladder by cutting the sacral posterior roots S2 to S4. A modification of this technique is the extradural implantation of the electrodes, a technique that is reserved for patients in whom arachnoiditis makes separation of the sacral roots impossible. The electrodes are implanted extradurally deeper on the sacral segmental nerves through a laminectomy of the sacrum only. This method is always an alternative in cases of irrepairable failures of the intrathecal implant or infection of the implant as in our patient. In some centres (e.g. Barcelona, Singapore, Ohio, Turin or Lisbon), this method is used for nearly all patients. In the situations of irrepairable failure of the extrathecal implant or its infection in combination with a previous intradural sacral deafferentation, there is no other alternative for implantation of the electrodes. In the situation of our patient, extrathecal approach was not available due to the previous extrathecal implantation (and secondary removal of the old device because of infection), and the sacral skin ulcerations and the intradural approach was also not feasible due to the previous intradural sacral deafferentation and the secondary arachnoiditis. The only alternative was the laparoscopic approach to the sacral roots. The field of the ‘laparoscopic neuro-functional pelvic surgery’ was developed by us.8 We routinely perform laparoscopic exposure and dissection of the endopelvic sacral nerve roots and of the pelvic splanchnic nerves during laparoscopic radical pelvic surgery,3 but also use the technique of ‘laparoscopic implantation of neuroprosthesis’ – LION procedure to cure patients of intractable pelveo-abdominal neuralgias.9 It was therefore proposed to the patient that the electrodes be implanted laparoscopically.
This laparoscopic approach presents some advantages when compared to the Brindley technique. First, there are the advantages related to the type of surgical procedure. As no approach from the spinal region is required (no laminectomy), there is no risk of meningitis, a smaller risk of infection and an even smaller risk of failure of the device, as the cables and electrodes do not run superficially under the skin but in a much safer place, implanted deep in the pelvis. There is also a smaller risk of wound healing problems as all the incisions are on the abdominal wall and are of small diameter. A second advantage is the facility of the surgical procedure. This procedure took approximately 3 h but we have demonstrated previously that we need an average of 14 min for laparoscopic exposure of the sacral roots on each side. We, therefore, firmly believe that with some technical improvements we will be able to perform this procedure in less than 1.5 h in the near future.
However, the most important aspect in the control of the bladder is undoubtedly not the voiding of the bladder but the reduction of the intravesical pressure both during storage and voiding. Classically, a bilateral posterior rhizotomy of S2 to S4 gives the best possible chance of achieving complete freedom from reflex incontinence with high pressure, and the greatest possible improvement in bladder compliance. This was not performed in this patient as she had undergone previously a sacral deafferentation. Thus, our technique can still not be considered as an alternative to the classical Brindley technique since we have never performed any sacral deafferentation by laparoscopic approach.
Conclusions
This case is the first report of a laparoscopic implantation of an endopelvic sacral neurostimulator for the recovery of bladder and intestinal functions in paralyzed patients. If the presented results could be reproduced in further patients and if a surgical or functional ‘deafferentation’ could also be obtained by minimal invasive way, the sacral laparoscopic implantation of a neuroprosthesis – sacral LION procedure – for recovery of bladder function in paralyzed patients could become a real alternative to the classical Brindley implantation. The presented sacral LION procedure for FES of the bladder and rectum has already been considered in patients after previous deafferentation with a non-functioning Brindley bladder controller as not only the surgical option to restore electrically induced miction when reimplantation by dorsal approach is contraindicated or unfeasible (patients after concomitantly intra- and extradural approach, after arachnoisdistis) but also as an alternative to be considered when reimplantation by dorsal way is possible since the laparoscopic approach is definitively less invasive (no laminectomy) and does not present any risk of arachnoditis or leakage of spinal fluid.
Conflict of interest
None.
References
Selzman AA, Hampel N . Urologic complications of spinal cord injury. Urol Clin North Am 1993; 20: 453–464.
Sidi AA, Becher EF, Reddy PK, Dykstra DD . Augmentation enterocystoplasty for the management of voiding dysfunction in spinal cord injury patients. J Urol 1990; 143: 83–85.
Possover M, Quakernack J, Chiantera V . The ‘LANN-technique’ to reduce the postoperative functional morbidity in laparoscopic radical pelvic surgery. J Am Coll Surg 2005; 6: 913–917.
Possover M, Rhiem K, Chiantera V . The ‘Laparoscopic Neuro-Navigation’ – LANN-technique: from a functional cartography of the pelvic autonomous neurosystem to a new field of laparoscopic surgery. Min Invas Ther and Allied Technol 2004; 13: 362–367.
Fawcett J . Repair of spinal cord injuries: where are we, where are we going? Spinal Cord 2002; 40: 615–623.
Gaunt RA, Prochazka A . Control of urinary bladder function with devices: successes and failures. Prog Brain Res 2006; 152: 163–194.
van Kerrebroeck PE, Koldewijn EL, Debruyne FM . Worldwide experiences with the Finetech-Brindley sacral anterior root stimulator. Neurourol Urodyn 1993; 12: 497–503.
Possover M . Laparoscopic exposure and electrostimulation of the somatic and autonomous pelvic nerves: a new method for implantation of neuroprothesis in paralysed patients? Journal Gynecological Surgery – Endoscopy, Imaging, and Allied Techniques 2004; 1: 87–90.
Possover M, Baekelandt J, Chiantera V . The Laparoscopic Implantation of Neuroprothesis – LION technique – to control intractable abdomino-pelvic neuralgia. Neuromodulation 2007; 10: 24–27.
Acknowledgements
No financial support was received for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Possover, M., Baekelandt, J., Kaufmann, A. et al. Laparoscopic endopelvic sacral implantation of a Brindley controller for recovery of bladder function in a paralyzed patient. Spinal Cord 46, 70–73 (2008). https://doi.org/10.1038/sj.sc.3102065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102065