Abstract
Study design: A cross-sectional study evaluating BMD at the hip and tibia, and SOS at the radius and mid-tibia in individuals with spinal cord injury (SCI) and a subgroup of non-SCI individuals.
Objectives: To investigate the speed of sound (SOS) in bone in relation to bone mineral density (BMD).
Setting: Kinesiology Department, McMaster University, Ontario, Canada.
Methods: In 14 individuals with SCI and 10 non-SCI individuals, proximal femur and tibia BMD were measured using dual energy X-ray absorptiometry, and radius and tibia SOS were measured with an ultrasonometer. T-scores were calculated using healthy reference databases. Inter-relationships between measurement techniques were determined using Pearson's correlation coefficients. P-values less than 0.05 were considered statistically significant.
Results: The average ages of the SCI and non-SCI groups were 33±9 and 27±6 years, respectively. Lesion level ranged from C4 to T12 and average time postinjury was 12 years, with a range of 1.6–25 years. Using the WHO criteria for osteoporosis, nine of 14 SCI subjects were osteoporotic at the hip, with the remainder in the osteopenic range. Tibia SOS T-scores were in the osteoporotic range for one subject with SCI, and two were in the osteopenic range. Among non-SCI individuals, one male had a tibia SOS T-score of −1.4, all others were within the normal range. Hip BMD and tibia SOS were significantly correlated (r=0.46, P<0.01). Hip BMD and tibia BMD were more strongly correlated (r=0.80, P<0.0005). Tibia BMD was not significantly correlated with SOS at the tibia (r=0.35, P=0.09). Radius SOS T-scores were positive and not significantly correlated with any lower limb variable.
Conclusion: Lower-limb bone mass is reduced in spinal cord-injured individuals, but SOS at the mid-tibia is not. It remains to be determined whether ultrasound measurements can predict fracture in the SCI population.
Similar content being viewed by others
Introduction
Lower-limb bone loss is an expected consequence following spinal cord injury (SCI). A cross-sectional study of bone changes following SCI estimated bone mass at the distal femur after an SCI to be 22, 27 and 37% lower than controls at 3, 4 and 16 months postinjury, respectively.1 A large cross-sectional study documented post-SCI bone density at the femoral neck, mid-shaft and distal femur to be 27, 25 and 43% below controls, respectively.2 Dramatic reductions in bone mass may predispose individuals with SCI to fracture, particularly in the lower limbs. A recent study demonstrated that among 41 men with SCI, 25 were osteoporotic and eight were osteopenic at the femoral neck according to the World Health Organization (WHO) criteria for osteoporosis, and 14 of these subjects had sustained a fracture after the SCI.3 Lower-limb fractures occurring in SCI individuals are often a result of trivial injuries or falls that would not normally cause a fracture, demonstrating the severity of osteoporosis.4 Delays in obtaining medical care after fracture, and/or misdiagnoses of fracture in the SCI population have been reported.4,5
The standard method of evaluating fracture risk and diagnosing osteoporosis is via measurements of hip and spine bone mineral density (BMD) using dual-energy X-ray absorptiometry (DXA). Accessibility to DXA machines is often limited to larger cities, so alternative methods for assessing bone status would be useful. Newer techniques, such as quantitative ultrasound (QUS) may provide a portable, more economical alternative to DXA that is also free of ionizing radiation. The Sunlight Omnisense Ultrasonometer (Sunlight Medical, Rehovot, Israel) is capable of measuring the speed of sound (SOS) conduction through bone at the phalanx, radius, tibia and metatarsal. The ability of the Omnisense to measure SOS at the tibia makes it an attractive alternative to DXA for osteoporosis screening, since it is portable, does not require the individuals to transfer onto a scanning table, and measures a site relevant for individuals with SCI. However, the ability of QUS to predict fracture is not as well established as for DXA, especially in the SCI population. The current study evaluated the relationship between hip BMD, as measured by DXA, and SOS, as measured by QUS, at the tibia and radius in individuals with chronic SCI. In order to evaluate the relationship between SOS and BMD across a large range of BMD values, a group of non-SCI individuals with normal bone mass was also included in the study. Since fractures of the proximal tibia are common in the SCI population,4 proximal tibia BMDs were also compared to tibia SOS. It was hypothesized that tibia SOS would be related to BMD measures at the hip and tibia, and that individuals with SCI would not only have low bone mass, but also have low SOS at the tibia.
Methods
Subjects
A total of 14 spinal-cord-injured subjects and 10 non-spinal-cord-injured subjects were recruited in accordance with the policies of the McMaster University Research Ethics Board. Informed consent was obtained prior to beginning the investigation. Exclusion criteria included the presence of metal implants in the measured leg or hip, or the possibility of pregnancy.
Protocol
SOS values at mid-tibia and distal third radius of each subject were measured using the Omnisense Ultrasonometer (Sunlight Ultrasound Technologies Ltd), which can be used to measure SOS at several skeletal sites. Ultrasonic waves at a frequency of 1.25 MHz are transmitted to the bone from a transducer in the ultrasound probe, and the speed of conduction through bone is measured. Software particular to the ultrasonometer digitizes and analyzes the signal, accounting for soft tissue. Long- and short-term precision for the Omnisense has been reported previously.6 For individuals with SCI who had lower-limb edema, two or three measurements at the tibia were often necessary in order to obtain a satisfactory signal.
Total proximal femur BMD and proximal tibia BMD were measured using DXA (Hologic 4500). Hip BMD was measured according to standard protocol. Tibia BMD was measured using the Hologic 4500 lumbar spine protocol, with modifications to the methods of analysis, described previously.7 The fibula was excluded from the analysis. The subject's lower leg was placed onto a specialized positioning device, which held the leg in 5° of knee flexion and approximately 10° of internal rotation. The scan distance was kept the same as that for the lumbar spine protocol. The scan was started at a point 20 cm below the superior aspect of the patella. The size of the region of interest to be analyzed was calculated relative to the width of the tibial epiphysis, so that the region of interest was proportional to body size.
Statistical analyses
Criteria analogous to those developed by the WHO for the interpretation of femoral neck BMD in postmenopausal women were applied to individual tibia and hip BMDs to categorize the participants as normal, osteopenic or osteoporotic.8,9 The Hologic 4500 contains a database of normal hip BMD values for a healthy reference population that were used for calculation of T-scores in the participants. The Sunlight Omnisense Ultrasonometer also contains a reference database of normal radius and tibia SOS values that were used for T-score calculation. For tibia BMD, we have collected sufficient reference data to calculate T-scores. Pearson's product moment correlations were calculated to determine if significant relationships existed between variables measured at the hip, radius and tibia using X-ray attenuation and ultrasound propagation. The P-values considered to be associated with statistical significance were those less than 0.05.
Results
The average age of the participants with SCI (11 male and three female participants) was 33±9 years, and the lesion levels ranged from C4 to T12. According to the American Spinal Injury Association (ASIA), the neurological impairment level for one individual was ASIA A, three were ASIA B, eight were ASIA C and two were ASIA D. The average time postinjury was 12 years, with a range of 1.6–25 years. The non-SCI (six male and four female) participants averaged 27±6 years of age.
Applying the WHO criteria for osteoporosis to the hip BMD data, nine of 14 SCI subjects were osteoporotic at the hip, and five were osteopenic. All of the non-SCI individuals had normal hip BMD according to WHO criteria. If the WHO criteria for osteoporosis were applied to the tibia BMD data, for the individuals with SCI, seven subjects would be considered osteoporotic, three would be osteopenic and the three others had T scores between −0.233 and −0.946. None of the non-SCI individuals had low tibia BMD. The average BMD data are presented in Table 1.
None of the subjects with SCI had a tibia SOS T-score in the osteoporotic range. Two subjects had osteopenic T-scores. Radius SOS T-scores were positive and averaged 0.74 and 1.1 in male and female subjects with SCI, respectively. Among the non-SCI individuals, one male had a tibia SOS T-score of −1.4, all others were within the normal range. The average SOS data are also presented in Table 1.
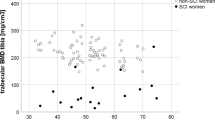
Hip BMD and tibia SOS were significantly correlated (r=0.46, P<0.024), as shown in Figure 1. Hip BMD and tibia BMD were more strongly correlated (r=0.83, P<0.0005), as shown in Figure 2. BMD at the tibia was not significantly correlated with SOS at the tibia (r=0.299, P=0.09, Figure 3). Radius SOS T-scores were not significantly correlated with any lower-limb variable (P>0.05).
Discussion
This study examined the inter-relationships between BMD at the hip and tibia and SOS at the tibia and radius in individuals with and without SCI. It is well established that individuals with SCI experience bone loss following injury, so it was not surprising that nine of the 14 (64%) individuals with SCI studied would be considered osteoporotic at the hip according to the WHO criteria. The remaining SCI subjects were in the osteopenic range. In addition, given the age range of the individuals without SCI, it was not surprising that their BMDs were normal according to the WHO criteria.
Most of the SOS T-scores of the individuals with SCI were within the normal healthy population ranges. This was somewhat surprising given the extent of lower-limb bone loss that occurs following an SCI. Also, one of the individuals without SCI had a high hip BMD and tibia BMD (total hip T-score +2.2, tibia T-score +2.6), yet for the same individual, the tibia SOS was lower than most of the individuals with SCI (T-score −1.4). Correlation coefficients revealed that, although hip and tibia BMD are correlated, tibia SOS is not correlated with tibia BMD and is only moderately correlated with hip BMD. The weak to moderate correlations found in this study are similar to those demonstrated previously.6,10
There are several possible explanations for the above findings. First, the lower-limb sites differ in location and bone type. SOS was measured at mid-tibia, whereas BMD measurements were taken at the proximal femur and proximal tibia. The proximal femur and proximal tibia sites contain both cortical and trabecular bone, whereas the mid-tibia site would be solely cortical bone. Second, the Omnisense Ultrasonometer measures the shortest path traveled by the ultrasound wave through the bone and, therefore, reflects the properties of the cortical shell. It has been suggested that tibia SOS measurements are influenced more by the density of the outermost layer of the cortex than by the inner regions.11 SOS is also dependent upon other bone properties, such as the structural organization of the bone, which will affect the relationship between BMD and SOS. Finally, it may not be appropriate to apply the WHO T-score criteria for osteoporosis to ultrasound measurements, or sites other than the proximal femur.12 In addition, the relationship between T-score and fracture risk may be different in the SCI population. The WHO T-score criteria for BMD measurements are based on the likelihood of hip fracture in postmenopausal women, and may only apply to that population, skeletal site and measurement technique.
The use of quantitative ultrasound for assessing the bone status in individuals with SCI is associated with a few practical limitations. The presence of lower-limb edema can make it difficult to obtain a signal at the tibia. Two or three measurements were required in a few subjects in order to mobilize some of the fluid to increase the contact between the probe and the bone surface. In addition, for the radius measurements, radius and phalangeal SOS measurements can be difficult in individuals with contractures and scar tissue. Finally, heterotopic ossification, which can occur after an SCI, may significantly interfere with BMD data. Heterotopic ossification was not an exclusion criterion for our study, and could potentially alter any associations between BMD and SOS measurements. However, that provides further argument for not using tibia SOS as a surrogate for tibia and hip BMD measurements in the SCI population.
Previous studies have demonstrated quantitative ultrasound to be sensitive to bone changes after SCI. In a cross-sectional study, ‘stiffness’ at the heel (a composite measure derived from broadband ultrasound attenuation (BUA) and SOS) was significantly lower in individuals with SCI than in a healthy reference population.13 Clearly, the calcaneus consists predominantly of trabecular bone and the structural organization may be an important variable contributing to the ultrasound measurement. A recent prospective study demonstrated that ultrasound measurements of SOS and BUA were sensitive to bone changes in the acute stages of SCI. The changes in BUA over a 6-week period during the first 6 months of injury were similar in magnitude to the changes in BMD at the calcaneus and tibia.14 However, both of these studies employed ultrasound measurements at the calcaneus, and the ultrasound devices used were different from each other and from the one used in the current study.
The Omnisense multisite ultrasound device can differentiate between pre- and postmenopausal women, and can distinguish individuals with hip or vertebral fracture from controls.6,15,16 However, the four measurement sites available with the multisite device (radius, phalanx, metatarsal and tibia) may not have the same ability to predict fracture. Unlike the other three sites, tibia SOS was not able to distinguish women with vertebral fracture from women without vertebral fracture.6 Another study measured a broader range of sites and all were able to discriminate subjects with hip fracture from controls, although the tibia was not included among the sites measured.16 Radius SOS was able to discriminate Colles' fracture cases from controls, however, the odds ratio was lower than for spine or hip BMD.17 SOS at the radius has also been demonstrated to be able to discriminate between elderly women with hip fracture and age-matched controls.18 Nevertheless, as with BMD measurements, there was some overlap in SOS values between the fracture and nonfracture groups. It is difficult to establish whether QUS is useful for osteoporosis screening in SCI when most studies investigating fracture risk have evaluated hip and vertebral fracture in non-SCI patients with osteoporosis. The ability of the multisite ultrasound device to predict femoral and tibial fractures, the most common sites of fracture in the SCI population, has not been established.
Conclusions
The results of this study confirm that individuals with SCI have low bone mass according to the WHO criteria for osteoporosis. Practical diagnostic techniques for the evaluation of fracture risk after SCI are essential. QUS is an attractive alternative to DXA because it is portable, patient-friendly and free of ionizing radiation. However, it may not be appropriate to use speed of sound measurements as a surrogate for BMD measurements in the SCI population. Future research should establish whether QUS demonstrates the ability to predict fracture risk in order to validate its use as a surrogate measure of bone status.
References
Garland DE et al. Osteoporosis after spinal cord injury. J Orthop Res 1992; 10: 371–378.
Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I . Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. J Rehabil Res Dev 2000; 37: 225–233 (in process citation).
Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M . Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 2001; 39: 208–214.
Ingram RR, Suman RK, Freeman PA . Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 1989; 27: 133–139.
Sobel M, Lyden JP . Long bone fracture in a spinal-cord-injured patient: complication of treatment — a case report and review of the literature. J Trauma 1991; 31: 1440–1444.
Knapp KM, Blake GM, Spector TD, Fogelman I . Multisite quantitative ultrasound: precision, age- and menopause-related changes, fracture discrimination, and T-score equivalence with dual-energy X-ray absorptiometry. Osteoporos Int 2001; 12: 456–464.
Moreno JC . Protocol for Using Dual Photon Absorptiometry Software to Measure BMD of Distal Femur and Proximal Tibia. McMaster University: Ontario, Canada 2001.
WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994; 843: 1–129.
Kanis JA . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368–381.
Foldes AJ, Rimon A, Keinan DD, Popovtzer MM . Quantitative ultrasound of the tibia: a novel approach for assessment of bone status. Bone 1995; 17: 363–367.
Prevrhal S et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporos Int 2001; 12: 28–34.
Faulkner KG, von Stetten E, Miller P . Discordance in patient classification using T-scores. J Clin Densitom 1999; 2: 343–350.
Chow YW et al. Ultrasound bone densitometry and dual energy X-ray absorptiometry in patients with spinal cord injury: a cross-sectional study. Spinal Cord 1996; 34: 736–741.
Warden SJ et al. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporos Int 2002; 13: 586–592.
Funck C et al. Ultrasound velocity of the tibia in normal German women and hip fracture patients. Calcif Tissue Int 1996; 58: 390–394.
Hans D et al. Does combining the results from multiple bone sites measured by a new quantitative ultrasound device improve discrimination of hip fracture? J Bone Miner Res 1999; 14: 644–651.
Knapp KM, Blake GM, Fogelman I, Doyle DV, Spector TD . Multisite quantitative ultrasound: Colles' fracture discrimination in postmenopausal women. Osteoporos Int 2002; 13: 474–479.
Weiss M, Ben Shlomo A, Hagag P, Ish-Shalom S . Discrimination of proximal hip fracture by quantitative ultrasound measurement at the radius. Osteoporos Int 2000; 11: 411–416.
Acknowledgements
Lora Giangregorio is the recipient of an Ontario Neurotrauma Foundation Studentship.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Giangregorio, L., Webber, C. Speed of sound in bone at the tibia: is it related to lower limb bone mineral density in spinal-cord-injured individuals?. Spinal Cord 42, 141–145 (2004). https://doi.org/10.1038/sj.sc.3101570
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3101570
Keywords
This article is cited by
-
Osteoporosis after spinal cord injury
Osteoporosis International (2006)