Abstract
To investigate the effects of fermented formula (FF) with Bifidobacterium breve C50 and Streptococcus thermophilus 065 on thymus size and stool pH of healthy term infants, ultrasound examinations and evaluations of thymus sizes and thymus indices (TI) and measurements of stool pH were performed in the same 90 term neonates on the 3rd d of life and on the 1st, 2nd, 3rd, and 4th mo of life. Thirty newborns were exclusively breast-fed while the remaining 60 were randomly assigned to receive either a FF or a standard formula (SF). The fecal pH of the breast-fed group was lower than the SF group (p < 0.05), although it was similar to that of the FF group on the third postnatal day, persisting for the entire 4 mo of the study. The difference in TI was statistically significant over repeated measurements among the groups. The FF infants showed a TI similar to the breast-fed newborns. Probiotic fermentation products have effects comparable to those of the bacteria composing the intestinal microflora supporting the idea that intestinal bacterial balance plays an important role in improving host immune responses.
Similar content being viewed by others
Main
The principal components of human milk modulating immune responses are immunoglobulin immune cells – macrophages, neutrophils, and lymphocytes – and humoral factors – lactoferrin, lysozyme, and hormones (1,2) that interact with the intestinal microflora during postnatal breast-feeding. The stool microflora of the breast-fed infant is predominantly composed of bifid-bacteria (3). The thymus provides the environment for T lymphocyte maturation. Sonographic studies show that the thymus grows until 8 mo of age decreasing to a stable size around 12 mo of age (4,5). Thymus size is dependent on whether the infant is breast-fed; the thymus size of breast-fed infants is twice the size of formula-fed infants at 4 mo of age (6). Now it is clearly known that infant feeding can modify the balance of intestinal flora and thus influence local and general immune response. One way to achieve this is using fermented formula, or formula containing probiotics or prebiotics (7,8). These products have been shown to have an effect on intestinal homeostasis and intestinal inflammation (9) and have been used to prevent and treat acute diarrhea. A new infant formula fermented with Bifidobacterium breve C50 and Streptococcus thermophilus 065 (FF) has been developed. These particular lactic-acid producing bacteria were selected because in vitro– in animal and human studies – they have been shown to have an effect on immune response and exert anti-inflammatory effects on gut-associated lymphoid tissue (GALT) (10,11). We hypothesized that fermented formula would result in a higher thymus index (TI) and a lower fecal pH over the first 4 months after birth mimicking the effects of breast milk.
MATERIALS AND METHODS
Inclusion and exclusion criteria.
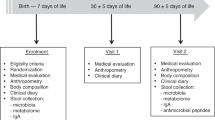
Over a period of 6 mo, healthy newborns born at the Department of Neonatology of the University of Bari were considered eligible to be included in the study. Newborns were selected on the basis of their parents' willingness to participate in the study. The exclusion criteria were prematurity, delivery by caesarian section, and any prenatal condition that could influence the thymus volume, such as maternal infection and under nutrition. Thirty newborns were exclusively breast-fed. Unfortunately, our efforts to convince the mothers to breast-feed were often unsuccessful. Information regarding the study was given only when the need of formula was imminent. Sixty newborns, whose mothers decided at their births not to breast-feed, were randomly assigned to receive either FF or standard infant formula (SF). These children did not receive colostrum. Both formulas had the same basic nutritional composition per 100 ml of 71 kilocalories, 1.5 g protein, 8.3 g carbohydrates, and 3 g fat.
Written informed consent was obtained from all parents and the study protocol was approved by the Ethics Committee of the Ospedale Consorziale Policlinico, Università di Bari. All newborns underwent a clinical examination and evaluation of weight, length, and head circumference at scheduled visits on the 3rd d of life. They were re-evaluated at 1, 2, 3, and 4 mo of age. Stools were collected at each visit. All the enrolled neonates completed the follow-up schedule. Physicians examining the infants and performing ultrasonographic evaluations were blind to the feeding regimen of the individual subject.
Ultrasonography measurement of the thymus was performed during each visit. The organ measurements were done by the operator using a Hewlett Packard Point HX and a 7.5 Mz probe. The thymus appears as a well-defined, echo-poor structure in the anterior mediastinum. The trans-sternal plane was used to measure the largest transverse diameter of the thymus; perpendicular to the diameter this large sagittal area (longitudinal scan plane) was depicted by the monitor and measured by the computer. The two measurements were multiplied and recorded as the TI and results were expressed as the average of two determinations. The index is an estimation of the volume of the thymus and postmortem examinations have shown a high correlation among thymus index, weight, and volume (12).
Fecal samples were collected as fresh as possible and immediately frozen at −20°C until evaluation. pH was measured using a Handy-lab pH meter (Schott Glas, Mainz, Germany) equipped with an Inlab 423 pH electrode (Mettler –Toledo Columbo). Fecal pH was determined after 10% fecal suspension (wt/vol) in saline solution (0.15 M NaCl solution).
Statistical analysis was performed using SPSS v 11.5 for Windows. The longitudinal study was designed to evaluate differences in growth parameters, TI and fecal pH with a power of 0.80 and a p value <0.05. ANOVA for repeated measures was used for TI and fecal pH among groups over time determination. Data were expressed as mean and SD.
The present investigation was conducted as a blind, randomized clinical trial to compare the effects of FF and breast milk on increasing TI and reducing fecal pH.
RESULTS
Demographic data are shown in Table 1. No differences in growth parameters, Apgar scores, or gender were seen among the three groups at the beginning of the study. The fecal pH of the breast-fed group was lower than that of the SF group (p < 0.05) while it was similar to that of the FF group on the 3rd postnatal day; this value persisted during the 4 mo of the study (Table 2).
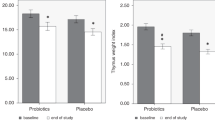
From the 1st postnatal evaluation and examination to the 3rd day of life, the TI of the breast-fed infants remained elevated compared with that of the FF and SF infants. The difference in TI was statistically significant over the repeated measurement among the BF and the other two groups (Fig. 1). The FF infants showed a TI similar to the breast-fed newborns (Fig. 1). None of the infants received antibiotics during the 4 mo of study.
Thymus Index of the three groups in different evaluations. BF (□; n = 30 newborns) FF (▪; n = 30 newborns) SF (□; n = 30 newborns).Values are expressed as mean and SD. TI increased over the time in a statistically different way in the three groups. BF vs SF (p < 0.001); BF vs FF p < 0.036; FF vs SF p < 0.042.
DISCUSSION
This randomized, double-blind study compares the effects of fermented formula with Bifidobacterium breve C50 and Streptococcus thermophilus 065 to breast milk and standard formula feeding on TI and fecal pH. The TI of breast-fed infants remained significantly higher than that measured in the formula-fed infants. Infants receiving FF showed a TI and a fecal pH similar to that of breast-fed babies compared with infants receiving SF. Moreover, the TI in BF newborns was larger from the 3rd d of life. Other studies in vivo reported the relation between the intestinal flora and general immune system (13). The study by Tribould found that babies provided with FF had an increase in bifid-bacteria associated with an increase in intestinal IgA antibody to poliovirus production after vaccination (14).
An increase in thymus size has already been reported in breast-fed infants (6). Breast milk contains a large number of immune components and some of these could be responsible for the modulation of the immune response (15). The role of the thymus in development and maturation of the newborn immune system plays a role in the differentiation and specialization of lymphoid cells populations. These cells will move toward other lymphoid districts and will contribute to tolerance against “self” antigens. Neuroendocrine regulation underlies adaptation to dynamic changes of the organism as environmental stimulations contribute to the full function of the thymus. It is widely known that the thymus plays a key role in the organic defense against infections and in the development of immune tolerance (16). Recently, it has been pointed out that the dimensions of the thymus at birth are associated with childhood mortality in developing countries. The determination of thymus dimensions at birth could be an important predictive factor of the immune competence level (17,18). Our data show that from the 3rd d of life breast-fed newborns had a TI significantly greater than those of the newborns in the other two groups. We speculated that this could be due to the effect of the components of the colostrums in breast milk that have stimulant effects on the immune system (19). We have no other explanation for this finding. The children belonged to the Caucasian race and the social factors in this group were homogeneous. Moreover, the breast-fed children persistently showed an increased TI. Jeppsen et al. (20) demonstrated an immune-modulating effect of breast milk with increases of CD4, CD8, and thymus index in breast-fed newborns relative to the formula-fed infants. This occurred from the 1st d of life until the 10th mo. The role of feeding in the development of the immune system, expressed incrementally by thymus volume, is clear. The same authors showed a decreased thymus size in uninfected infants of HIV-positive mothers fed on human donor milk compared with that of exclusively breast-fed newborns. The results are larger than that of the formula-fed infants group. This difference was clearly seen at 4 mo even though at birth those children had a small thymus size compared with infants of HIV-negative mothers (21). These data support the hypothesis that changes in intestinal microflora “related to different type of feeding” might influence the thymus size. Contrary to Hasselbach, Jeppesen, and our study data where the feeding clearly influences the dimensions of the thymus, independent of other clinical variables such as weight, length, and head circumference, Yekeler (22) demonstrated no correlation between thymus dimensions and type of feeding. A low power of the study (i.e., a small number of formula-fed infants compared with the breast-fed controls) might explain these findings.
The relatively higher growth of the thymus in the breast-fed babies may also be explained by many variables. First, the increased production of CD4 and CD8 demonstrated from recent studies suggest that the larger size of the thymus is compatible with increased thymic activity and emission of T lymphocytes (23). Moreover even if the fetal immune system could be influenced during gestation, the composition of human milk plays a crucial role in the development of the infant immune system via several cytokines and growth factors (24). Bioactive IL-10 in human milk has immune-modulating, anti-inflammatory effects on the alimentary tract (25) and a protective role against necrotizing enterocolitis (26). IL-7, an essential factor for proliferation and survival of T cell precursors has been found in high concentrations in human milk from the first lactating week (27). Other studies underline the importance of IL-7 in conditioning T cell function outside the thymic environment as well (28,29).
The observation that TI is larger in the FF group compared with SF infants deserves further comment. Bacterial species similar to those used to ferment the study formula secrete moieties that retain anti-inflammatory properties after crossing an in vitro model of intestinal barrier (10). Therefore, unidentified soluble factors might explain the immune regulatory properties of the FF. As a complementary hypothesis, the fermentation process itself might influence the composition of intestinal microflora and, in turn, the infant immune system. Although we did not document a direct change in intestinal microflora composition, we demonstrate an acidic shift in fecal pH. This is indirect though reliable evidence of an increasing lactobacillar stool concentration (30,31). We are aware of the limitations of this method, which does not allow us to draw any conclusion about the specific composition of intestinal microflora. New techniques are emerging, such as those which use 16S ribosomal RNA (rRNA) gene sequences or strain specific Rep-PCR for evaluation of microbial populations (32,33). The modification of fecal pH associated with alteration of TI in our studies is a phenomenon that deserves further evaluation using these emerging technologies.
The active metabolites from this formula are of interest for newborns that are at higher risk of infections due to live bacteria, such as in premature infants with an underdeveloped immune system. Understanding the mechanism of the beneficial effect of the FF on endogenous microflora and immunogenic activities should provide new regimens for prevention and treatment of illness without the potential detrimental effects of giving live microorganisms. In conclusion, probiotic fermentation products may have, as in this case, beneficial effects comparable to those of the bacteria, which compose the optimal intestinal microflora. This strengthens the idea that intestinal bacterial balance plays an important role in improving host immune responses to different situations.
Abbreviations
- BF:
-
breast-fed
- FF:
-
fermented formula
- GALT:
-
gut associated lymphoid tissue
- SF:
-
standard formula
- TI:
-
thymus index
References
Hanson LA 1999 Human milk and host defence: immediate and long-term effects. Acta Paediatr 88: 42–46
Howie PW, Forsyth JS, Ogston SA, Clark A, Florey CD 1990 Protective effect of breast feeding against infection. BMJ 300: 11–16
Beerens H, Romond C, Neut C 1980 Influence of breast-feeding on the bifid flora of the newborn intestine. Am J Clin Nutr 33: 2434–2439
Hasselbalch H, Jeppesen DL, Ersboll AK, Engelmann MD, Nielsen MB 1997 Thymus size evaluated by sonography. A longitudinal study on infants during the first year of life. Acta Radiol 38: 222–227
Hasselbalch H, Ersboll AK, Jeppesen DL, Nielsen MB 1999 Thymus size in infants from birth until 24 months of age evaluated by ultrasound A longitudinal prediction model for the thymic index. Acta Radiol 40: 41–44
Hasselbalch H, Jeppesen DL, Engelmann MD, Michaelsen KF, Nielsen MB 1996 Decreased thymus size in formula-fed infants compared with breastfed infants. Acta Paediatr 85: 1029–1032
Rautava S, Arvilommi H, Isolauri E 2006 Specific probiotics in enhancing maturation of IgA responses in formula-fed infants. Pediatr Res 60: 221–224
Collins MD, Gibson GR . Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 1999 69: 1052S–1057S
Szajewska H, Mrukowicz JZ 2001 Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 33: S17–S25
Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M 2004 Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53: 821–828
Romond MB, Ais A, Yazourh A, Romond C 1997 Cell-free Wheys From Bifidobacteria Fermented Milks Exert a Regulatory Effect on the Intestinal Microflora of Mice and Humans. Anaerobe 3: 137–143
Hasselbalch H, Nielsen MB, Jeppesen D, Pedersen JF, Karkov J 1996 Sonographic measurement of the thymus in infants. Eur Radiol 6: 700–703
Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R 2001 Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322: 1327
Thibault H, Aubert-Jacquin C, Goulet O 2004 Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J Pediatr Gastroenterol Nutr 39: 147–152
Goldman AS 1993 The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J 12: 664–671
Thestrup-Pedersen K, Dwyer JM, Askenase PW 1977 Studies on the role of the thymus and T cells in the in vivo suppression of delayed hypersensitivity (desensitization): radiosensitivity of the mechanism inducing nonspecific anergy. J Immunol 118: 1665–1671
Prentice AM 1999 The thymus a barometer of malnutrition. Br J Nutr 81: 345–347
Savino W 2002 The thymus gland is a target in malnutrition. Eur J Clin Nutr 56: S46–S49
Kelly D, Coutts AG 2000 Early nutrition and the development of immune function in the neonate. Proc Nutr Soc 59: 177–185
Jeppesen DL, Hasselbalch H, Lisse IM, Ersboll AK, Engelmann MD 2004 T-lymphocyte subsets, thymic size and breastfeeding in infancy. Pediatr Allergy Immunol 15: 127–132
Jeppesen D, Hasselbalch H, Ersboll AK, Heilmann C, Valerius NH 2003 Thymic size in uninfected infants born to HIV-positive mothers and fed with pasteurized human milk. Acta Paediatr 92: 679–683
Yekeler E, Tambag A, Tunaci A, Genchellac H, Dursun M, Gokcay G, Acunas G 2004 Analysis of the thymus in 151 healthy infants from 0 to 2 years of age. J Ultrasound Med 23: 1321–1326
Olesen AB, Andersen G, Jeppesen DL, Benn CS, Juul S, Thestrup-Pedersen K 2005 Thymus is enlarged in children with current atopic dermatitis. A cross-sectional study. Acta Derm Venereol 85: 240–243
Garofalo RP, Goldman AS 1999 Expression of functional immunomodulatory and anti-inflammatory factors in human milk. Clin Perinatol 26: 361–377
Garofalo R, Chheda S, Mei F, Palkowetz KH, Rudloff HE, Schmalstieg FC, Rassin DK, Goldman AS 1995 Interleukin-10 in human milk. Pediatr Res 37: 444–449
Fituch CC, Palkowetz KH, Goldman AS, Schanler RJ 2004 Concentrations of IL-10 in preterm human milk and in milk from mothers of infants with necrotizing enterocolitis. Acta Paediatr 93: 1496–1500
Ngom PT, Collinson AC, Pido-Lopez J, Henson SM, Prentice AM, Aspinall R 2004 Improved thymic function in exclusively breastfed infants is associated with higher interleukin 7 concentrations in their mothers' breast milk. Am J Clin Nutr 80: 722–728
Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN 1998 IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol 161: 5909–5917
Seddon B, Tomlinson P, Zamoyska R 2003 Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol 4: 680–686
Langhendries JP, Detry J, Van Hees J, Lamboray JM, Darimont J, Mozin MJ, Secretin MC, Senterre J 1995 Effect of a fermented infant formula containing viable bifidobacteria on the faecal flora composition and pH of healthy full-term infants. J Pediatr Gastroenterol Nutr 21: 125–129
Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, Boehm G 2002 Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr 34: 291–295
Waters SM, Murphy RA, Power RF 2006 Characterisation of prototype Nurmi cultures using culture-based microbiological techniques and PCR-DGGE. Int J Food Microbiol 110: 268–277
Elli M, Callegari ML, Ferrari S, Bessi E, Cattivelli D, Soldi S, Morelli L, Goupil Feuillerat N, Antoine JM 2006 Survival of yogurt bacteria in the human gut. Appl Environ Microbiol 72: 5113–5117
Acknowledgements
The authors thank Professor Joseph Neu and Dr. Francesco Raimondi for their helpful suggestions, and Dr. Ssa Marilù Lattarulo for revising the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was supported by a Mellin SpA grant.
Rights and permissions
About this article
Cite this article
Indrio, F., Ladisa, G., Mautone, A. et al. Effect of a Fermented Formula on Thymus Size and Stool pH in Healthy Term Infants. Pediatr Res 62, 98–100 (2007). https://doi.org/10.1203/pdr.0b013e31806772d3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/pdr.0b013e31806772d3
This article is cited by
-
The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics
Nature Reviews Gastroenterology & Hepatology (2021)
-
Thymus size in children with moderate malnutrition: a cohort study from Burkina Faso
Pediatric Research (2021)
-
Effect of probiotics on thymus size and markers of infection in late infancy: a randomized controlled trial
Pediatric Research (2021)
-
Breast milk interleukin-7 and thymic gland development in infancy
European Journal of Nutrition (2020)
-
Fermented infant formulas without live bacteria: a systematic review
European Journal of Pediatrics (2015)