Abstract
We hypothesized that early infancy would be a time of oxidative stress due to the difficulty of adapting to ambient oxygen. Therefore, we measured levels of products of lipid peroxidation (F2-isoprostanes), antioxidant enzyme activity (catalase (CAT) and superoxide dismutase (SOD)), and ability to resist oxidative stress (ferric reducing ability of plasma (FRAP)) in full-term infants (38–42 wk) fed human milk from birth. Seventy-seven infants were followed at 1, 3.5, 6, and 12 mo of age. F2-isoprostanes in plasma declined significantly (p < 0.05) from 1 to 6 mo (160 ± 43; 90 ± 33; 41 ± 27 pg/mL (mean ± SD)). FRAP values (775 ± 196, 723 ± 133, 697 ± 126, 669 ± 145 μM) 1, 3.5, 6, and 12, respectively) declined (p = 0.06) from 1 to 3.5 mo and from 3.5 to 6 mo of age. RBC-SOD (2.7 ± 2, 3.2 ± 2.8, 2.1 ± 1.8, 2.5 ± 1.8 U, 1, 3.5, 6, 12 mo, respectively) declined from 3.5 to 6 mo. RBC-CAT (76 ± 23, 94 ± 28, 81 ± 22, 85 ± 31 U, 1, 3.5, 6, 12 mo, respectively) also declined between 3.5 and 6 mo, after a significant increase between 1 and 3.5 mo. These data suggest that the human infant is under oxidative stress early in infancy and further study may be warranted to assess the potential benefits of antioxidant supplementation for either the mother or the infant.
Similar content being viewed by others
Main
The process of childbirth is accompanied by an increase in oxidative stress, as birth is, in itself, a hyperoxic challenge. The fetus transfers from an intrauterine hypoxic environment with a pO2 of 20–25 mm Hg to an extrauterine normoxic yet relatively hyperoxic environment with a pO2 of 100 mm Hg (1). Increased exposure to oxygen at relatively high concentrations, compared with the womb can be accommodated by neonatal animals of many species because of the newborn lungs' ability to increase its normal complement of protective antioxidant enzymes during O2 exposure (2). The evolutionary adaptation to extra uterine aerobic existence required the development of efficient cellular electron transport systems to produce energy. In concert with this challenge to energy-producing oxidative metabolism, biochemical defenses including antioxidant enzymes, evolved to protect against oxidation of cellular constituents by oxygen radicals (3–5).
Not only do these antioxidant enzymes mature during late gestation (6) but there is increased transfer of antioxidants across the placenta, including vitamins E, C, beta-carotenes, and ubiquinone during the last days of gestation (7,8). While attention has been focused on pathologic diseases in newborns, particularly the premature infant (9,10) either from immaturity or impairment of antioxidant enzymes or an increase in the production of reactive oxygen species (ROS) due to elevated intakes of O2, few data exist about the neonatal adaptation to physiologic stress of delivery and early postnatal life in full-term healthy infants.
Clearly ROS play a role in signal transduction and are essential for development (11). How the newborn infant can cope with possible excess exposure to ROS is not yet clear. It is possible that developing antioxidant defense mechanisms may be overcome by the generation of excessive ROS during the neonatal period (12). We and others have shown that human milk provides antioxidant protection in early life with the direct ability to scavenge free radicals, not seen in artificial infant feeds (13,14). Indeed, van Zoeren-Grobben reported that infants fed human milk had higher plasma trapping ability, a measure of resistance to oxidative stress in vitro, than did control infants who were formula fed (15). This may be due to the presence of antioxidant enzymes glutathione peroxidase (GPx), catalase (Cat), and superoxide dismutase (SOD) present in human milk, but not in formula (16), which in addition to their antioxidant effect in the gut may pass through the porous neonatal intestine early in infancy (13).
We hypothesized that early infancy would be a time of oxidative stress due to the difficulty of adapting to ambient oxygen. Therefore, as part of a larger study on iron metabolism we assessed lipid peroxidation, activity of antioxidant enzymes, and the ability to resist oxidative stress in full-term healthy breast-fed infants during the 1st year of life. As a measure of lipid peroxidation we measured F2-isoprostanes, which are prostaglandin F2-like compounds produced by free-radical catalyzed peroxidation of arachidonic acid (17). Measurements of F2-isoprostanes are considered the most reliable approach to assess oxidative stress in vivo. To examine a comprehensive set of measures of oxidative stress we assayed activities of SOD, CAT, and the ferric reducing ability of plasma (FRAP) assay as a measurement of overall ability to resist oxidative stress.
METHODS
Subjects.
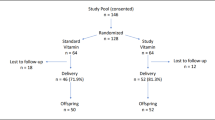
Between January 1999 and August 2000, mother-infant dyads were approached in the regional postpartum unit in St. John's, Newfoundland, for informed consent to enter this study. The primary requisite for inclusion was intent to breast-feed exclusively (with no more than one supplemental feed per day for at least 4 mo), as per recommendations (18). Exclusion criteria included gestation <37 wk, birthweight <2.5 kg, multiple pregnancy, major illness requiring intensive care admission, and major congenital anomaly. Seventy-seven dyads that were successfully breast-feeding at 1 mo were recruited and a subset was studied. Parents' educational status, socioeconomic status, and other demographic data were ascertained at study entry.
Methods.
Blood samples were drawn by venipuncture (1 mL) into heparinized vacutainer tubes at study entry (1 mo ± 2 d) and follow-up clinics at 3.5, 6, and 12 mo (± 1 wk). Samples were analyzed for red blood cell superoxide dismutase and catalase by spectrophotometric measurements (19,20); ferric reducing ability of plasma (FRAP) according to Benzie and Strain (21) using standard procedures from our laboratory. Plasma F2-isoprostanes were measured by gas chromatography mass spectrometry (17). Not enough blood was available for all analyses. Anthropometric data were obtained at each clinic visit.
Data analysis.
Continuous variables were analyzed using repeated measures ANOVA, t tests and Pearson correlation coefficients (SPSSx v9.0). Nominal variables were compared by χ2 test. Significance (two-tailed) was assigned at p < 0.05.
Ethical approval was obtained from the Human Investigation Committee of Memorial University of Newfoundland and informed consent was obtained from parents/guardians of each infant.
RESULTS
Dropout rates.
Seventy-seven, 56, 51, and 44 infants were seen at 1, 3.5, 6, and 12 mo for blood collection, respectively. The number of samples analyzed at 1 mo for FRAP, CAT, and SOD was n = 73, for F2-isoprostanes n = 12; at 3.5 mo for FRAP, CAT, and SOD n = 48, for F2-isoprostanes n = 32; at 6 mo for FRAP, CAT and SOD n = 37, for F2-isoprostanes n = 6; at 12 mo, FRAP, CAT, and SOD n = 27. At 1 mo, all infants were exclusively breast-fed except three infants, receiving about 125 mL formula/d. At month 3.5, 14 infants received supplemental feedings of about 125 mL/d. By 6 mo, the majority of infants received formula and by 12 mo most infants received cow's milk, or formula alone.
F2-isoprostane levels (Fig. 1) declined significantly from month 1 to 3.5 months, and from 3.5 months to 6 mo. FRAP (Fig. 2) showed a trend to decline over the same time period. The levels of F2-isoprostanes at 1 month were markedly elevated compared with levels measured in normal adults (35.5 ± 6.1, mean ± SD) (17,22). CAT activity (Fig. 3) showed a significant rise between 1 and 3.5 mo of age and a subsequent decline between 3.5 and 6 mo. SOD activity (Fig. 4) declined significantly between 3.5 and 6 mo of age and were higher than levels measured in normal adults (0.8 ± 0.01 (22)). No biochemical variables were related to either parental data or infant anthropometric data (not shown).
DISCUSSION
The unexpected finding from our study was the markedly elevated levels of F2-isoprostanes in plasma in early infancy (Fig. 1). The rapid decline in F2-isoprostanes between 1 and 3 and 3 and 6 mo to normal adult levels (35.5 ± 6.1, (17,23)) suggests that these infants have slowly adjusted to oxidative stress over time. In support of these findings both SOD and CAT increased between 1 and 3 mo and then declined, suggesting a response to oxidative stress that appears to be ameliorated with age.
Further support for a response to oxidative stress comes from the FRAP assay. This assay measures in vitro the global ability of the infant to resist an oxidative challenge. Levels from month 1 declined thereafter, and at no time in infancy did FRAP values reach normal adult levels (1017 ± 206 μM (21). As FRAP measures the global ability to resist oxidative stress, and F2-isoprostanes reflect the effects of oxidative stress resulting in tissue damage, high F2-isoprostanes at month 1 and a declining FRAP value throughout infancy suggest that normal healthy full-term infants are coping with oxidative stress early in life. The most likely candidates, although additional factors may be involved for this stress, are the transition from a hypoxic environment in the womb to a normoxic but relatively hyperoxic extrauterine environment and a high metabolic rate requiring a high level of mitochondrial respiration and subsequent enhanced mitochondrial superoxide formation (23). Because of the high levels of inspired oxygen required to maintain arterial oxygen tension necessary for post natal life, which are substantially higher than those that would normally be present during fetal existence, newborns are far more exposed to reactive oxygen species (ROS) than they would be had they remained in utero. That this transition is stressful can be seen from supportive evidence concerning the mortality rate in the first 28 d of life compared with the remainder of infancy (24). In the United States, 67% of all deaths during the 1st y occur in the 1st mo of life. This mortality data suggests that the transition from the womb to the extrauterine environment is an oxidative challenge, that may overwhelm the antioxidant capacity of the organism (7) and that not all infants can successfully cope with this event. That this challenge involves an oxidative stress in coping with ambient oxygen pressure has been shown in several studies (25–28).
There are numerous reports in the literature of oxidative stress associated with birth and that normal labor is associated with oxidative stress for the neonate. Higher lipid peroxidation reflected by increased malondialdehyde levels (MDA) in cord blood, than were found in the neonatal period suggests oxidative stress during the birth process (25–27). Two further studies reported increased MDA in newborn infants compared with adults (28,29). Berger et al. (30) reported higher levels of F2 isoprostanes at birth in preterm infants compared with adults, suggesting that premature birth is associated with even greater oxidative stress. Elevated F2 isoprostanes in children under 6 mo of age compared with older children were attributed to both a higher frequency of infections in children fewer than 3 y associated with a higher metabolic rate (31).
Collectively these data suggest that newborn infants are experiencing oxidative stress that resolves only with age. Possible mechanisms may include the degradation of fetal erythrocytes that are present in early infancy. In vitro, fetal erythrocytes produce more superoxide and hydrogen peroxide than do adult red blood cells (32). It is known that during the fetal-neonatal transition period, dramatic changes are occurring in the pO2 in lung and blood cells with a more gradual change in liver and brain (33). These changes may result in increased oxidative stress to cells.
Further, Gonzalez et al. (33) reported that changes in antioxidant defenses could be related to the beginning of food intake after birth, which entails higher hepatic metabolism rate as well as oxygen consumption. While we did not study bottle-fed infants from birth, some of our infants switched to formulas during the first 6 mo. There were no differences in any of our measurements according to type of feed. Others (34) reported that bottle-fed infants from 2–4 mo of age had lower MDA than breast-fed infants and attributed these findings to higher levels of long chain fatty acids, present in human milk but not formulas. In the latter study (34) there was no difference in the in vitro ability to resist oxidative stress, suggesting that these infants could cope with this oxidative stress, as was seen in our study. Recently, infant formulas have been fortified with DHA and EPA, long chain fatty acids (Mead Johnson, Evansville, IN). The effect on oxidative stress seen in early infancy by formula-fed infants with these new supplements remains to be determined.
As with other populations undergoing oxidative stress including premature infants (9) and diabetics (35), one is tempted to intervene with dietary supplements. Full-term healthy breast-fed infants are routinely given vitamin K supplements at birth, and vitamin D supplements during infancy (36). Indeed it was once common to give vitamin D as part of a multivitamin solution containing vitamin A (an antioxidant) as well as vitamin K (Michael Moffatt, personal communication). We hypothesize that it would not be unreasonable to consider antioxidant supplements in early infancy at least for selected groups of breast-fed infants including those of multiple pregnancies or infants from low socioeconomic backgrounds. We know that formula-fed infants consume greater amounts of vitamins and minerals than are present in human milk (37,38) without any known untoward effects. Alternatively, mothers could be supplemented with additional antioxidant nutrients during pregnancy to enhance the endogenous ability of the infant at birth to cope with oxygen stress. In either case, the goal of supplementation would be to reduce oxidative stress (decreased F2-isoprostanes) and increase the endogenous ability of the infant to resist oxidative stress (maintain a higher FRAP).
We repeat that these are hypotheses, particularly as we did not follow infants during the 1st month of breast-feeding in the current study. As well, a high O2 tension is required for maturation (39) and free radicals are essential as cell signaling molecules (40,41). The benefit of supplementation would be uncertain, as it appears to be the imbalance of response to oxidative stress that is problematic rather than oxidative stress itself.
Abbreviations
- CAT:
-
catalase (EC 1.11.1.6)
- FRAP:
-
ferric reducing ability of plasma
- RBC:
-
red blood cells
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase (EC 1.15.1.1)
- SD:
-
standard deviation
References
Muller DP 1987 Free radical problems of the newborn. Proc Nutr Soc 46: 69–75
Frank L 1985 Effects of oxygen on the newborn. Fed Proc 44: 2328–2334
Frank L, Sosenko IR 1987 Prenatal development of lung antioxidant enzymes in four species. J Pediatr 110: 106–110
Frank L, Groseclose EE 1984 Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res 18: 240–244
Vlessis AA, Mela-Riker L 1989 Perinatal development of heart, kidney and liver mitochondrial antioxidant defense. Pediatr Res 26: 220–226
Gerdin E, Tyden O, Eriksson UJ 1985 The development of antioxidant enzymatic defense in the perinatal rat lung: activities of superoxide dismutase, glutathione peroxidase, and catalase. Pediatr Res 19: 687–691
Robles R, Palomino N, Robles A 2001 Oxidative stress in the neonate. Early Hum Dev 65: S75–S81
Ripalda MJ, Rudolph N, Wong SL 1989 Developmental patterns of antioxidant defense mechanisms in human erythrocytes. Pediatr Res 26: 366–369
Friel JK, Widness JA, Jiang T, Belkhode SL, Rebouche CJ, Ziegler EE 2002 Antioxidant status and oxidant stress may be associated with vitamin E intakes in very low birth weight infants during the first month of life. Nutr Res 22: 55–64, 2002
Lindeman JH, van Zoeren-Grobben D, Schrijver J, Speek AJ, Poorthuis BJ, Berger HM 1989 The total free radical trapping ability of cord blood plasma in preterm and term babies. Pediatr Res 26: 20–24
Allen RG 1991 Oxygen-reactive species and antioxidant responses during development: The metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med 196: 117–129
Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA 1994 Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett 349: 197–200
Friel JK, Martin SM, Langdon M, Herzberg GR, Buettner GR 2002 Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res 51: 612–618
Buescher ES, McIlherhan SM 1988 Antioxidant properties of human colostrum. Pediatr Res 24: 14–19
van Zoeren-Grobben D, Lindeman JH, Houdkamp E, Brand R, Schrijver J, Berger HM 1994 Postnatal changes in plasma chain-breaking antioxidants in healthy preterm infants fed formula and/or human milk. Am J Clin Nutr 60: 900–906
L'Abbe MR, Friel JK 1992 Copper status of very low birth weight infants during the first 12 months of infancy. Pediatr Res 32: 183–188
Morrow JD, Roberts LJ 2nd 1999 Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol 300: 3–12
Canadian Pediatric Society 1991 Meeting the iron needs of infants and young children: An update. CMAJ 144: 1451–53
Oberley LW, Spitz DR 1984 Assay of superoxide dismutase in tumor tissue. Methods Enzymol 105: 457–464
Claiborne A 1985 Catalase activity. In: Greenwald RA (ed) CRC Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton pp 283–284
Benzie IF, Strain JJ 1999 Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299: 15–27
Delmas-Beauvieux MC, Combe C, Peuchant E, Carbonneau MA, Dubourg L, de Precigout V, Aparicio M, Clerc M 1995 Evaluation of red blood cell lipoperoxidation in hemodialysed patients during erythropoietin therapy supplemented or not with iron. Nephron 69: 404–410
McCord JM 2000 The evolution of free radicals and oxidative stress. Am J Med 108: 652–659
MacDorman MF, Minino AM, Strobino DM, Guyer B 2002 Annual summary of vital statistics-2001. Pediatrics 110: 1037–1052
Nycyk JA, Drury JA, Cooke RW 1998 Breath pentane as a marker for lipid peroxidation and adverse outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed 79: F67–F69
Rogers MS, Mogelli JM, Tsang KH, Wang CC, Law KP 1998 Lipid peroxidation in cord blood at birth: the effect of labour. Br J Obstet Gynaecol 105: 739–744
Buonocore G, Zani S, Perrone S, Caciotte B, Bracci R 1998 Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hyperoxic newborn. Free Radic Biol Med 25: 766–770
Wispe JR, Bell EF, Roberts RJ 1985 Assessment of lipid peroxidation in newborn infants and rabbits by measurements of expired ethane and pentane: influence of parenteral lipid infusion. Pediatr Res 19: 374–379
McCarthy K, Bhogal M, Nardi M, Hart D 1984 Pathogenic factors in bronchopulmonary dysplasia. Pediatr Res 18: 483–488
Berger TM, Polidori MC, Dabbagh A, Evans PJ, Halliwell B, Morrow JD, Roberts LJ 2nd, Frei B 1997 Antioxidant activity of vitamin C in iron-overloaded human plasma. J Biol Chem 272: 15656–15660
Weinsier RL, Schutz Y, Bracco D 1992 Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr 55: 790–794
Jain SK 1989 The neonatal erythrocyte and its oxidative susceptibility. Semin Hematol 26: 286–300
Gonzalez MM, Madrid R, Arahuetes RM 1995 Physiological changes in antioxidant defenses in fetal and neonatal rat liver. Reprod Fertil Dev 7: 1375–1380
Granot E, Golan D, Rivkin L, Kohen R 1999 Oxidative stress in healthy breast fed versus formula fed infants. Nutr Res 19: 869–879
Akkus I, Kalak S, Vural H, Caglayan O, Menekse E, Can G, Durmus B 1996 Leukocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase and serum leukocyte vitamin C levels of patients with type II diabetes mellitus. Clin Chim Acta 244: 221–227
Fomon SJ 2001 Infant feeding in the 20th century: formula and beikost. J Nutr 131: 409S–420S
Friel J 2003 The nutritional composition of human milk and infant formula. In: Wilson T, Temple N (edt) Beverages for the Food Industry. Humana Press, Totowa NJ pp 235–246
Friel JK, Andrews WL, Simmons BS, Mercer C, McDonald A, McCloy UR 1997 Evaluation of full-term infants fed an evaporated milk formula. Acta Paediatr 86: 448–453
Allen RG, Venkatraj VS 1992 Oxidants and antioxidants in development and differentiation. J Nutr 122( 3 Suppl) 631–635
Spitzer JA 1995 Active oxygen intermediates—beneficial or deleterious? An introduction. Proc Soc Exp Biol Med 209: 102–103
Droge W 2002 Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95
Acknowledgements
The authors thank Claude Mercer and Allison McDonald for technical help, and Dr. W.L. Andrews and Dr. K. Aziz for help with recruitment. As well, the parents of infants and the nurses in the NICU contributed greatly to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support was received from the Janeway Research Foundation; Canadian Institutes of Health Research grant #146833; and grants GM42056, GM15431, DK26657 from the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Friel, J., Friesen, R., Harding, S. et al. Evidence of Oxidative Stress in Full-Term Healthy Infants. Pediatr Res 56, 878–882 (2004). https://doi.org/10.1203/01.PDR.0000146032.98120.43
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000146032.98120.43
This article is cited by
-
A synbiotic intervention modulates meta-omics signatures of gut redox potential and acidity in elective caesarean born infants
BMC Microbiology (2021)
-
N-Acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets
European Journal of Nutrition (2019)
-
HDAC4 preserves skeletal muscle structure following long-term denervation by mediating distinct cellular responses
Skeletal Muscle (2018)
-
N-acetylcysteine attenuates intrauterine growth retardation-induced hepatic damage in suckling piglets by improving glutathione synthesis and cellular homeostasis
European Journal of Nutrition (2018)
-
Can the preterm lung recover from perinatal stress?
Molecular and Cellular Pediatrics (2016)