Abstract
The purpose of this study was to determine the time of onset, duration, and the efficacy of in vivo gene transfer in protecting the ornithine transcarbamylase deficient spf/Y mouse from an acute ammonium challenge. The animals were challenged with ammonia (10 mmol/kg NH4Cl) 1, 2, 7, 14, or 28 d after the administration of a recombinant adenoviral construct deleted in E1 and with a temperature sensitive mutation in E2. Although there was no protection with the control LacZ virus, the ornithine transcarbamylase (OTC)-containing vector provided partial protection from both behavioral symptoms (ataxia, seizures, and abnormal response to sound) and biochemical abnormalities (ammonium, aspartate, alanine, and glutamine) within 24 h and complete protection by 48 h. Mortality was also decreased. Animals receiving the vector 7 and 14 d before the ammonium load were also protected, whereas those treated 28 d before the challenge were not. OTC enzyme activity in liver of untreated spf/Y mice was 5% of control C3H mice. After gene transfer, activity was increased to near control levels through 14 d but had returned to baseline by 28 d. These studies indicate that adenovirus-mediated gene transfer confers a metabolic benefit within 24 h of administration and provides protection against an acute metabolic insult for at least 2 wk.
Similar content being viewed by others
Main
OTC (EC 2.1.3.3) is a mitochondrial enzyme, primarily expressed in liver, that participates with four other enzymes in the urea cycle. An inherited deficiency of OTC compromises ureagenesis leading to protein intolerance and potentially life-threatening hyperammonemia. Clinical manifestations of this X-linked recessive disorder appear in hemizygous males and in some heterozygous females(1). Age of onset and severity of symptoms correlate with residual enzyme activity in liver. Approximately 60% of hemizygous males have no measurable OTC activity and develop lethargy, hypotonia, seizures, and coma in association with greater than 10-fold elevations in plasma ammonium in the first few days of life(2, 3). The other 40% of hemizygous males have a mutation causing a partial OTC deficiency (5-15% normal activity) and develop episodes of less severe hyperammonemia at times of excess protein intake or catabolic stress later in childhood or adulthood(4). A similar clinical pattern of partial OTC deficiency is seen in about 10% of heterozygous females who have disproportionate lyonization of the X chromosome carrying the normal OTC allele(5, 6).
Despite recent advances in acute treatment using hemodialysis and in long-term therapy involving the stimulation of alternate pathways of waste nitrogen excretion using sodium phenylbutyrate, OTC deficiency remains a devastating disease(1). The mortality rate for neonatal hyperammonemic coma approaches 50%, whereas each subsequent hyperammonemic crisis carries a 10% mortality rate(7). Among survivors there is a correlation between the duration of neonatal hyperammonemic coma and subsequent neurodevelopmental deficits. Greater than three-quarters of infants in coma for more than 3 d have been found to develop mental retardation and other disabilities(8).
As a result of the poor prognosis, there have been recent attempts at permanent correction of OTC deficiency via orthotopic liver transplantation(9–11). This has been successful in correcting most of the metabolic derangements in the few patients studied, although it carries its own significant risks. Its success suggests that liver directed gene therapy also may be effective. Furthermore, if gene expression could be induced rapidly, within 1-2 d of onset of a hyperammonemic crises, it might influence neurodevelopmental outcome. This report describes a study to determine the rapidity of metabolic correction and clinical protection after in vivo gene transfer with an adenoviral vector in an animal model of OTC deficiency, the sparse fur (spf/Y) mouse.
Two animal models of OTC deficiency, the spf and spfash mouse, have been used to study pathogenesis and to evaluate novel therapies(12–14). The spf strain has a missense mutation that renders the OTC enzyme catalytically defective, whereas the spfash mouse has a mutation that alters mRNA splicing and diminishes the level of OTC mRNA. Both models retain 5-15% residual OTC enzyme activity in liver and have a mild phenotype that resembles humans with partial OTC activity (i.e., late onset hemizygotes and symptomatic heterozygotes). The mice display many of the metabolic alterations observed in children with OTC deficiency, including elevated plasma ammonium and glutamine, decreased plasma citrulline, and increased urinary orotic acid(14).
A number of groups have studied gene transfer in the spf and spfash mice, most commonly using adenoviral vectors. The adenovirus was chosen because of its tropism for hepatocytes, thereby permitting in vivo liver-directed gene transfer. Previous studies using E1-deleted adenoviral vectors expressing reporter genes demonstrated a high level of recombinant gene expression in liver of a variety of species(15–19). However, experiments in adult animals were complicated by destructive cellular and humoral immune responses leading to inflammation, loss of transgene expression, and difficulties with vector readministration(20, 21).
The first evaluation of adenoviral vectors in the spf mouse were performed by Stratford-Perricaudet et al.(22) in newborn mice, whose immune responses are blunted due to neonatal tolerance. The characteristic cutaneous manifestations of the spf phenotype and urinary orotate excretion were partially corrected. Morsy et al.(23) subsequently confirmed these observations. Two groups extended this approach to the treatment of adult spf animals that more closely resemble newborn humans in their immunologic maturity(24, 25). In one of these studies, we reported that a second generation adenoviral vector expressing murine OTC, with a temperature-sensitive mutation at E2 in addition to the E1 deletion, led to less immune activation and achieved metabolic correction for up to 2 mo in the adult spf/Y mouse(25). In these studies with adult animals, high levels of OTC activity were observed within a few days of injection of the adenoviral vector, but 7-14 d were required for the normalization of steady state metabolic measures. Using the second generation vector, in the current study we sought to determine how quickly gene transfer is protective against an acute nitrogen challenge in spf/Y and control C3H mice.
METHODS
Construction and propagation of the second generation recombinant adenoviruses. The second generation recombinant adenovirus carrying mouse OTC cDNA was constructed as described previously(25). Briefly, mouse OTC cDNA was generated by reverse transcription-polymerase chain reaction, cloned into a pGEM-T vector (Promega, Madison, WI), and restricted with SpeI and SacII. A 1.5-kb fragment containing mouse cDNA was isolated, blunted, and cloned into EcoRV site of an adenoviral vector pAd.CMV-link1(25). The new plasmid, designated pAd.CMVmOTC, was linearized with EcoRI and contransfected into 293 cells with ClaI/XbaI-restricted Ad5 viral DNA containing the ts125 mutation in E2a(26) and the sub360 mutation in E3(27). The resulting recombinant virus, designated H5.110CMV mOTC [for nomenclature of recombinant adenoviruses generated in Institute for Human Gene Therapy, University of Pennsylvania, see Engelhardt et al.(28)], was purified through three rounds of plaque isolation. The second generation β-galactosidase (LacZ) virus(H5.110CMV/LacZ) was constructed as described previously(19) and purified through three rounds of plaque isolation. Each virus was titered by a standard plaque-forming assay. The batches of virus used in the current study had 40 particles per PFU. The dose of virus used in the current study is based on our previous analysis of the dose-response relationship for this virus(25). The dose used in the current study was one-half that used for many of the previous metabolic studies to minimize possible toxicity.
spf/Y mouse colony. The spf mouse colony used in these studies was originally obtained from Jackson Laboratories and has been backcrossed to C3HeB/J (C3H) mice for more than 20 generations. The colony was maintained on normal rodent diet (Teklad sterilizable rodent diet (W) 8656, North Penn Feeds Inc., Lansdale, PA). Animals had access to food ad libitum before and during the study. To produce spf/Y and littermate controls, heterozygote females were mated with wild type C3HeB/J. Spf/ Y and male C3HeB/J progeny from this cross (6-10 wk of age) were used in this study.
Gene transfer. Blood samples were collected by retroorbital bleeding 3 d before viral administration (day -3). On d 0, virus (1 × 1011 PFU/kg) suspended in 0.1 mL of PBS was administered to animals via the tail vein. The animals were then challenged with NH4Cl on d 1, 2, 7, 14, or 28 after gene transfer. Although some mice were challenged more than once, no mice were challenged more than three times, and mice challenged on d 1 were a different group from those challenged on d 2.
Ammonium challenge. To simulate a nitrogen challenge, mice(control and virus-treated) were injected with a 0.64 M solution of NH4Cl intraperitoneally. All ammonium challenges were performed at the same time of day, between 1100 and 1300 h, to minimize the effect of food consumption on plasma amino acids. At 15-20 min after injection, the mice were evaluated behaviorally and scored by two blinded observers using the scale outlined in Table 1. Blood was collected from the retro-orbital plexus of the mice 20 min after injection. The samples were immediately processed and analyzed for ammonium and amino acids.
Behavioral scoring system. A behavioral scoring system was developed to quantify the manifestations of acute hyperammonemia in the mouse after a nitrogen load. The scoring system was based on the appearance of ataxia (A), seizures (S), and abnormal response to sound (R). These criteria are summarized in Table 1. Ataxia was determined by gently pulling on the mouse's tail and observing gait. A score of 2 was assigned if the mouse was able to ambulate normally; a score of 1 was assigned if the mouse staggered away; a score of 0 signified the mouse was unable to walk. Seizures were categorized as spontaneous myoclonus or tonic-clonic movements. Finally, hyperresponsiveness to sound was determined by ringing a 100-db bell 5-6 times in series and observing mouse behavior.
Based on this scoring system, a normal mouse would be expected to receive a score of seven (A2S2R3). Severely affected mice would receive a score of one (A0S1R0). Any mice that died during the challenge automatically received a score of zero. Mice that exhibited tonic-clonic seizures always died after the seizures. Scoring was performed by two different observers (C.P. and T.Q. or C.P. and X.Y.) who were blinded to treatment as described in each of the figure legends. Because C3H and spf/Y mice are generally distinguishable based on appearance, observers could not be blinded to type of mouse. The inter-rater reliability of this scoring system was examined in preliminary studies by Spearman correlation, correlation = 0.95, p < 0.0001, n = 17 observations.
Metabolic measures. Plasma ammonium levels were determined with a Kodak Ektachem DT60 system. Plasma amino acids were analyzed by HPLC after precolumn derivitization with o-phthaldialdehyde as previously described(29). Briefly, after centrifugation of heparinized blood, an aliquot of plasma was immediately precipitated with an equal volume of 0.8 N perchloric acid, which contained the internal standards L-α-aminoadipate and L-α-amino-n-butyric acid. After centrifugation, an aliquot of the supernatants was neutralized with 2 M KHCO3. These samples were derivitized using an autosampler. External standards were injected after every fifth specimen. OTC activity in liver was measured as described by Lee and Nussbaum(30) with modifications. Briefly, 2-10 μg of total cellular protein were added to 700μL of reaction mixture (5 mM ornithine, 15 mM carbamyl phosphate, and 270 mM triethanolamine, pH 7.7), which was incubated at 37 °C for 30 min. Reactions were stopped by adding 250 μL of 3:1 phosphoric acid/sulfuric acid (by volume). Citrulline production was then determined by adding 50 μL of 3% 2,3-butanedione monoxime, incubating at 95-100 °C in the dark for 15 min, and measuring absorbance at 490 nm.
Statistical analyses. Analyses were performed to address the following questions. 1) Does the NH4Cl challenge alter the specific measure (behavioral score or level of metabolite) in spf/Y or C3H mice that were not treated with adenovirus? 2) Does the LacZ virus affect the control mouse? 3) Does either the LacZ or the OTC virus alter the measure in the spf/Y mouse? The statistical analyses were performed using SPSS (Statistical Package for Social Sciences Version PC 6.01, Inc., Chicago, IL). Plasma ammonium and amino acids levels were log-transformed because of skewed distributions. Analyses of variance were used for group comparisons of these log-transformed data. The nonparametric Kruskal-Wallis one-way ANOVA was used to compare behavioral scores in the groups of mice. Postanalysis of variance comparisons were examined by pairwise t tests for the plasma ammonium and plasma amino acid levels. Postanalysis of variance comparisons were examined by pairwise Mann-Whitney tests for the nonparametric behavioral scores. Bonferroni corrections were used for these pairwise comparisons.
RESULTS
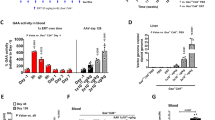
Hepatic OTC activity in spf/Y mice after administration of the OTC adenoviral vector. Analysis of liver tissue lysates from spf/Y mice treated with the OTC vector at various times after the gene transfer demonstrated the following levels of OTC enzyme activity relative to that found in control C3H animals: baseline spf/Y (3.0 ± 0.7μmol citrulline/mg protein/h, n = 6), baseline C3H (63.9 ± 6.0μmol citrulline/mg protein/h, n = 7), d 1 spf/Y (28.1± 9.8 μmol citrulline/mg protein/h, n = 4), d 2spf/ Y (72.1 ± 22.0 μmol citrulline/mg protein/h,n = 5), d 7 spf/Y (52.4 ± 3.4 μmol citrulline/mg protein/h, n = 2), d 14 spf/Y (38.0 ± 9.1 μmol citrulline/mg protein/h, n = 4), and d 28 spf/Y (1.6± 0.0 μmol citrulline/mg protein/h, n = 2).
Effect of ammonium challenge on nitrogen metabolism. Amino acids and ammonium were measured in blood collected before and 20 min after challenge with 10 mmol/kg NH4Cl or saline (Table 2). Consistent with previous reports(14) baseline(saline-injected) plasma ammonium and some amino acids were significantly different in spf/Y and C3H mice not treated with the vector. Concentrations of plasma ammonium (0.13 mM in spf/Y versus 0.058 mM in C3H, p = 0.0006) and glutamine (0.74 versus 0.43 mM, p < 0.0001) were significantly higher in the spf/ Y mice. Plasma citrulline (0.019 versus 0.055 mM,p < 0.0001) and arginine (0.066 versus 0.095 mM,p < 0.001) were significantly lower in the spf/Y mice.
In C3H mice, the 10 mmol/kg NH4Cl challenge resulted in an increase in plasma ammonium levels from 0.058 mM (n = 5) to 1.8 mM(n = 12; p < 0.0001 by Mann-Whitney U Wilcoxon rank sum test). The only significant changes in amino acids were a 50% increase in citrulline (p = 0.001) and a 35% decrease in glutamate (p < 0.0001). In spf/Y mice, plasma ammonium increased from 0.13 mM (n = 5) to 7.8 mM (n = 10) after the NH4Cl challenge (p < 0.0001). Effects on amino acids included a 3-fold increase in alanine (0.59 versus 1.8 mM,p < 0.0001), a 5-fold increase in aspartate (0.015versus 0.078 mM, p = 0.002), and a 2-fold increase in glutamate (0.043 versus 0.095 mM, p < 0.0001)(Table 2).
LacZ adenoviral vector had little effect on nitrogen metabolism after an ammonium challenge. There was concern that the adenovirus itself might adversely affect nitrogen metabolism through a mechanism of liver inflammation or other perturbations in physiology. C3H mice and spf/ Y mice were therefore administered an adenovirus construct containing the LacZ reporter gene and challenged with NH4Cl (10 mmol/kg) at subsequent times; results were compared with C3H or spf/ Y animals that had received no virus before the load(Table 3, Fig. 1). In the NH4Cl challenged spf/ Y mice, there were no significant differences between untreated spf/ Y and LacZ-treated spf/Y mice in plasma amino acids or ammonium levels at 1, 2, 7, or 14 d. At 28 d, the levels of glutamine in the LacZ-treated animals were higher than those observed in untreated spf/ Y mice after NH4Cl challenge (0.93 versus 0.66 mM, p = 0.001). The LacZ virus had modest effects on metabolic parameters in C3H mice at 7 d.
Plasma ammonium levels measured 20 min after challenge in spf/Y and C3H control mice. spf/Y or C3H mice (6-10 wk of age) were injected with 1 × 1011 PFU/kg of recombinant adenovirus containing mouse OTC cDNA (H5.110CMV mOTC) or LacZ(H5.110CMVLacZ) in 0.1 mL of PBS via the tail vein. spf/Y and C3H mice were injected with PBS only and were included in each experiment. The animals were injected with NH4Cl (10 mmol/kg) at 2 or 7 d post vector infusion. Blood was collected 20 min after injection with NH4Cl and ammonium was analyzed in the plasma. Data are the antilog of the logarithmic mean with confidence intervals of the antilog of log mean + 2 SD and - 2 SD of between 4 and 12 independent observations. After log normalization of ammonium levels, data were compared by ANOVA. Plasma ammonium in NH4Cl challenged C3H mice were compared with plasma ammonium in NH4Cl challenged C3H mice that had been treated with the LacZ virus. Plasma ammonium in NH4Cl challenged spf/Y mice were compared with plasma ammonium in NH4Cl challenged spf/Y mice that had been treated with either the LacZ or OTC virus (*p = 0.002;**p = 0.001).
OTC adenoviral vector prevents accumulation of nitrogen metabolites after an ammonium challenge. The effects of LacZ- and OTC-containing adenoviral constructs on plasma ammonium levels after ammonium challenge were compared (Fig. 1). There were overall group differences in the levels of ammonium at d 2 (p = 0.002) and at d 7 (p < 0.0001). Individual comparisons revealed significantly more accumulation of plasma ammonium in untreated spf/ Y than in OTC-treated spf/Y mice challenged with ammonia either 2 d after (p = 0.004) or 7 d after (p = 0.001) treatment. In fact, after ammonium challenge the levels of ammonium in the OTC treated spf/Y mice and in C3H mice were not significantly different. Comparison of untreated spf/Y mice with spf/Y mice receiving the OTC virus revealed higher levels of alanine (p< 0.0001), aspartate (p < 0.0001), glutamate (p = 0.005), and lysine (p = 0.03) in the untreated group. With these overall group differences, we searched for differences at each of the time points after virus injection. Compared with untreated spf/Y mice,spf/ Y mice challenged with ammonia 2, 7, and 14 d after gene transfer showed less accumulation of alanine, glutamate, and aspartate (see Table 3). Compared with untreated spf/Y mice, OTC-treated spf/Y mice challenged with ammonia 2, 14, and 28 d after gene transfer showed less accumulation of lysine (p < 0.01, data not shown). Although there were overall group differences in the levels of citrulline (p = 0.005) and arginine (p < 0.0001), when comparing OTC treated spf/Y mice with untreated spf/Y mice, there were no significant differences at any of the individual time points.
A model for ammonium induced behavioral changes in spf/Y mice. Individual cohorts of spf animals were injected with single doses of NH4Cl ranging from 4 to 10 mmol/kg and observed using the behavioral scoring system outlined in Table 1.Figure 2 presents the relationship between the dose of NH4Cl and behavior of the mice. There was a direct correlation between the dose of NH4Cl and severity of symptoms in the spf/Y mice. These ranged from essentially no symptoms (mean score = 6.7 ± 0.2) at 4 mmol/kg to virtually universal mortality (mean score = 0.6 ± 0.2) at 10 mmol/kg (Spearman correlation = -0.847, p < 0.0001, n = 43). C3H mice challenged with identical doses of NH4Cl remained asymptomatic at all doses except for 10 mmol/kg where a few animals demonstrated ataxia (mean score 5.9 ± 0.3, Spearman correlation =-0.360, p = 0.02, n = 40). Mann-Whitney nonparametric statistics were used to compare the effects of NH4Cl challenge on C3H and spf mice at each dose. There were significant differences in behavioral scores between spf/Y and C3H mice at 6 (p = 0.004), 8 (p = 0.005), and 10 mmol/kg (p < 0.0001)(Fig. 2).
Dose response to ammonia challenge in spf and control C3H mice. spf or C3H mice (6-10 wk of age) were injected intraperitoneally with NH4Cl at indicated doses. Scores were based on behaviors observed between 15 and 20 min after injection of NH4Cl as outlined in Table 1. Observers were blinded to the dose of NH4Cl. Data are the mean ± SEM of between 6 and 16 independent observations with different mice. Open circles, C3H mice; closed circles, spf/Y mice. Mann Whitney-Wilcoxon rank sum test was used to test for differences in response of C3H and spf/Y mice at each dose (*p = 0.004; **p = 0.005;***p = 0.0001).
The LacZ adenoviral vector had little effect on behavior after an ammonium challenge. The behavioral scores were also evaluated in C3H and spf/ Y mice treated with the LacZ vector to determine whether this construct had a significant effect on control or spf/Y mice. The C3H mice treated with the LacZ virus were indistinguishable from nonvector-treated mice with scores of 6.1 ± 0.5 (n = 8) after an ammonium challenge (10 mmol/kg) given 2 d after gene transfer and 5.2 ± 0.5(n = 14) 7 d after gene transfer. spf/Y mice treated with the LacZ virus demonstrated behavioral abnormalities after ammonium challenge that were not significantly different from that observed in untreated spf animals after nitrogen challenge (Fig. 3).
Behavioral consequences of ammonium challenge in spf/ Y mice treated with adenoviral vectors. spf/Y mice(6-10 wk of age) were injected with 1 × 1011 PFU/kg of recombinant adenovirus containing mouse OTC cDNA (H5.110CMV mOTC)(closed circles), or LacZ (H5.100CMVLacZ) (closed squares), in 0.1 mL of PBS via the tail vein. Control mice (spf/Y and C3H) were injected with PBS only and were included in each experiment. The animals were injected with NH4Cl (10 mmol/kg) at different times after vector infusion. Response to ammonium challenge was observed and scored as described in Table 1. Observers were blinded to treatment(type of recombinant adenovirus or PBS) before ammonium challenge. A Mann Whitney-Wilcoxon rank sum test was used to compare scores for control spf/ Y to adenovirus-injected spf/Y (*p = 0.01, **p = 0.001, ***p = 0.0005,****p < 0.0001). Because of the multiple comparisons (5) a p < 0.01 is considered significant. Data are the mean ± SEM.
The OTC adenoviral vector prevented behavioral sequelae after an ammonium challenge. C3H and spf/Y mice were evaluated for NH4Cl-induced behavioral abnormalities after gene transfer. C3H mice challenged with 10 mmol/kg NH4Cl received a score of 5.9 ± 0.2(n = 18) (where 7 is unaffected and 1 is moribund). Spf/Y mice receiving an ammonium challenge of 10 mmol/kg without gene transfer demonstrated behavioral scores of 1.3 ± 0.6 (n = 9). Pretreatment with the adenoviral OTC vector ameliorated these abnormalities (Fig. 3). A statistically significant improvement in behavioral score was detected in spf/Y mice as soon as 1 d after gene therapy compared with untreated spf/Y mice, 5.0 ± 0.9(n = 6) versus 1.3 ± 0.6 (n = 9;p = 0.01). This improvement was also observed 2 (6.0 ± 0.5,n = 10, p = 0.0005), 7 (6.0 ± 0.5, n = 15, p < 0.0001), and 14 d (5.7 ± 0.5, n = 6,p = 0.002) after treatment with the OTC adenoviral vector(comparison by Mann-Whitney U Wilcoxon rank sum test). These behavioral scores of spf/Y mice receiving the OTC adenoviral construct were statistically indistinguishable from control C3H mice. spf/ Y pretreated 28 d before the ammonium load did not benefit; their scores did not differ significantly from spf/Y mice that had not received gene therapy (3.3 ± 0.8 versus 1.3 ± 0.6,p = 0.06).
DISCUSSION
The principal focus of research into in vivo gene therapy for human disease has been on prolonging the expression of the transgene. In this study, we focused instead on the rapidity of action of gene transfer in a disorder in which prevention or rapid treatment of a metabolic disturbance is essential to avert irreversible brain damage. The OTC-deficient spf/ Y mouse was used in the study. We initially compared the effects of the ammonium challenge on metabolism and behavior in untreated spf/ Y and control C3H mice. We found that the response to an ammonium challenge was quite different in these two groups of mice. In terms of metabolic changes, plasma ammonium levels rose much less in the C3H mice than in the spf/Y mice. The plasma levels of citrulline were increased, glutamate was decreased, and glutamine was not altered in the C3H mice. These results are compatible with previous reports that a nitrogen challenge induces urea synthetic activity(31). In the spf/ Y mouse, with ureagenic capacity estimated to be 60% of control based on metabolic flux of [15N]NH4Cl into [15N]urea in vivo(14), the ammonium challenge caused a significant accumulation of alanine, glutamate, and aspartate and had no effect on citrulline. This suggests that the urea synthetic capacity had been exceeded by the nitrogen load. Although it would require experiments beyond the scope of the current study to explain the differences in the response of C3H and spf/Y mice to an ammonium challenge, the elevations of alanine, glutamate, and aspartate are observed in patients with OTC deficiency and other urea cycle disorders during an acute hyperammonemic crisis(32, 33). The similarity in amino acid alterations observed in the NH4Cl challenged spf/Y mouse to those observed in these patients provides support for this being a reasonable model of an acute hyperammonemic crisis.
With regard to the effects of the ammonium challenge on behavior, there also was a significant difference between the untreated spf/Y and C3H mice. The spf/Y mice were much more sensitive to the nitrogen load, showing a dose-response curve with most animals becoming moribund from a challenge of 10 mmol/kg NH4Cl. The C3H mice showed virtually no change in behavior until the highest dose (10 mmol/kg) and then only manifested transient ataxia.
With these significantly different metabolic and behavioral responses to an NH4Cl challenge, it was possible to assess the rapidity and efficacy of adenoviral mediated gene transfer in providing protection against these effects. We found that gene transfer of an OTC-containing recombinant adenovirus was partially protective against an ammonium load as early as 24 h after administration. Pretreatment 48 h before the challenge resulted in levels of plasma ammonium and amino acids which did not rise above those observed in control C3H mice. There was also a normalization of the behavioral response to the ammonium challenge. The protection lasted at least through d 14 after gene transfer. In a previous study of adenoviral mediated OTC gene transfer, we had observed correction of plasma glutamine and urinary orotate for at least 42 d(25). In the present study, there was a return of OTC activity to baseline levels and no metabolic or clinical protection against the ammonium challenge at 28 d indicating a loss of gene expression, possibly as a consequence of an immune response. This apparent difference in length of efficacy may be related to the different measures used to assess efficacy, or to the slightly lower dose of virus used in the present study (1 × 1011 versus 2 × 1011 PFU/kg).
The observation that there was substantial protection through d 14 is important in relation to the potential toxic effects of the adenoviral vector. Previous studies have shown that recombinant adenoviruses elicit a brisk immune response 7-14 d after injection, leading both to inflammation of the liver and loss of gene expression(20, 21). We have also observed direct liver toxicity 24 h and 3 d after viral infusion(our unpublished observations). The early and late toxicity are partially blunted with the second generation vector. One could postulate that an adverse response to the vector could actually place the spf/Y mouse at increased risk for metabolic derangement and clinical deterioration from a nitrogen load as a result of liver damage and resultant impairment of residual OTC activity. To test this hypothesis, we administered an ammonium challenge both to spf/Y mice that had received an adenoviral construct containing a reporter LacZ gene and to spf/Y mice who had received no virus. The behavioral and metabolic responses were comparable in these two groups, other than a somewhat increased accumulation of glutamine in the LacZ-treated mice at 28 d. Although these findings do not exclude immune-related liver toxicity, they suggest that any adverse effect may be counterbalanced by increased OTC activity.
In sum, this study provides evidence that in vivo gene transfer using an adenovirus vector can rapidly protect the OTC-deficient spf/ Y mouse from developing fatal hyperammonemia after a nitrogen load. Protection was present within 24 h of gene transfer and lasted at least 14 d. This suggests the possibility that in vivo gene therapy may eventually prove useful as part of the acute treatment of hyperammonemia in children with OTC deficiency.
Abbreviations
- OTC:
-
ornithine transcarbamylase
- spf:
-
sparse fur
- spfash:
-
sparse fur-ash
- LacZ:
-
β-galactosidase
- PFU:
-
plaque-forming unit
- ANOVA:
-
analysis of variance
References
Brusilow SW, Horwich AL 1995 Urea cycle enzymes. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, pp 1187–1232
Batshaw ML 1994 Inborn errors of urea synthesis. Ann Neurol 35: 133–141
Nagata N, Matsuda I, Matsuura T, Oyanagi K, Tada K, Narisawa K, Kitagawa T, Sakiyama T, Yamashita F, Yoshino M 1991 Retrospective survey of urea cycle disorders. Part 2. Neurological outcome in forty-nine Japanese patients with urea cycle enzymopathies. Am J Med Genet 40: 477–481
Finkelstein JE, Hauser ER, Leonard CO, Brusilow SW 1990 Late-onset ornithine transcarbamylase deficiency in male patients. J Pediatr 117: 897–902
Arn PH, Hauser ER, Thomas GH 1990 Hyperammonemia in women with a mutation at the ornithine carbamoyltransferase locus. N Engl J Med 322: 1652–1655
Batshaw ML, Msall M, Trojak J, Beaudet AL 1986 Risk of serious illness in heterozygotes of ornithine transcarbamylase deficiency. J Pediatr 108: 236–241
Batshaw ML, Brusilow S, Waber L, Blom W, Brubakk AM, Burton BK, Cann HM, Kerr D, Mamunes P, Matalon R, Myerberg D, Schafer IA 1982 Treatment of inborn errors of urea synthesis: activation of alternative pathways of waste nitrogen synthesis and excretion. N Engl J Med 306: 1387–1392
Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED 1984 Neurologic outcome in children with inborn errors of urea synthesis: outcome of urea-cycle enzymopathies. N Engl J Med 310: 1500–1505
Todo S, Starzl TE, Tzakis A, Benkov KJ, Kalousek F, Saheki T, Tanikawa K, Fenton WA 1992 Orthotopic liver transplantation for urea cycle enzyme deficiency. Hepatology 15: 419–422
Tuchman M 1989 Persistent acitrullinemia after liver transplantation for carbamylphosphate synthetase deficiency. N Engl J Med 320: 1498–1499
Largilliere C, Houssin D, Gottrand F, Mathey C, Checoury A, Alagille D, Farriaux J 1989 Liver transplantation for ornithine transcarbamylase deficiency in a girl. J Pediatr 115: 415–417
Veres G, Gibbs RA, Scherer SE, Caskey CT 1987 The molecular basis of the sparse fur mouse mutation. Science 237: 415–417
Hodges PE, Rosenberg LE 1989 The spfash mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc Natl Acad Sci USA 86: 4142–4146
Batshaw ML, Yudkoff M, McLaughlin B, Gorry E, Anegawa NJ, Smith IA, Hyman SL, Robinson MB 1995 The sparse fur mouse as a model for gene therapy in ornithine carbamoyltransferase deficiency. Gene Ther 2: 743–749
Jaffe H 1992 Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nature 1: 372–378
Herz J, Gerard RD 1993 Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci USA 90: 2812–2816
Li Q, Kay M, Finegold M, Stratford-Perricaudet L, Woo S 1993 Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther 4: 403–409
Kay MA, Landen CN, Rothenberg SR, Taylor LA, Leland F, Wiehle S, Fang B, Bellinger D, Finegold M, Thompson AR, Read M, Brinkhous KM, Woo SLC 1994 In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci USA 91: 2353–2357
Engelhardt J, Ye X, Doranz B, Wilson J 1994 Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA 91: 6196–6200
Yang Y, Ertl H, Wilson J 1994 MHC Class 1 restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1 deleted recombinant adenoviruses. Immunity 1: 433–442
Yang Y, Nunes F, Berencsi K, Furth E, Gonczol E, Gønczøl E 1994 Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA 91: 4407–4411
Stratford-Perricaudet LD, Levrero M, Chasse JF, Perricaudet M, Briand P 1990 Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther 1: 241–256
Morsy M, Zhao J, Ngo T, Warman A, O'Brien W, Graham F, Caskey C 1996 Patient selection may affect gene therapy success: dominant negative effects observed for ornithine transcarbamylase in mouse and human hepatocytes. J Clin Invest 97: 826–832
Kiwaki K, Kanegae Y, Saito I, Komaki S, Nakamura K, Miyazaki J-I, Endo F, Matsuda I 1996 Correction of ornithine transcarbamylase deficiency in adult spfash mice and in OTC-deficient human hepatocytes with recombinant adenoviruses bearing the CAG promoter. Hum Gene Ther 7: 821–830
Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I, Wilson JM 1996 Prolonged metabolic correction in adult ornithine transcarbamylase deficient mice with adenoviral vectors. J Bio Chem 271: 3639–3646
Ensinger MJ, Ginsberg HS 1972 Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol 10: 328–339
Logan J, Shenk T 1984 Adenovirus tripartite leader sequence enhances translation of mRNAs late after injection. Proc Natl Acad Sci USA 81: 3655–3659
Engelhardt J, Litzky L, Wilson J 1994 Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum Gene Ther 5: 1217–1229
Robinson MB, Djali S, Buchhalter JR 1993 Inhibition of glutamate uptake with L-trans-pyrrolidine-2,4-dicarboxylate potentiates glutamate toxicity in primary hipocampal cultures. J Neurochem 61: 2099–2103
Lee JT, Nussbaum RL 1989 An arginine to glutamine mutation in residue 109 of human ornithine transcarbamylase completely abolishes enzymatic activity in Cos1 cells. J Clin Invest 84: 1762–1766
Stewart PM, Walser M 1980 Short term regulation of ureagenesis. J Biol Chem 255: 5270–5280
Bachmann C 1974 Urea cycle. In: Nyhan WL (ed) Heritable Disorders of Amino Acid Metabolism: Patterns of Clinical Expression and Genetic Variation. John Wiley & Sons, New York, pp 361–386
Batshaw ML, Monahan PS 1987 Treatment of urea cycle disorders. Enzyme 38: 242–250
Acknowledgements
The authors thank Linda Crnic from the University of Colorado for advice in developing the scoring system used to evaluate the response on the mice to an NH4Cl challenge.
Author information
Authors and Affiliations
Additional information
Supported in part by grants (HD32649 and HD26979) from the National Institutes of Health (X.Y., J.M.W., M.B.R., M.L.B.). Support was also provided by Genovo, Inc., which J.M.W. consults for and holds equity in, and by the Vector Discovery and Production Program and Clinical Pathology Unit of the Institute for Human Gene Therapy.
Rights and permissions
About this article
Cite this article
Ye, X., Robinson, M., Pabin, C. et al. Adenovirus-Mediated in Vivo Gene Transfer Rapidly Protects Ornithine Transcarbamylase-Deficient Mice from an Ammonium Challenge. Pediatr Res 41, 527–534 (1997). https://doi.org/10.1203/00006450-199704000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199704000-00012
This article is cited by
-
Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering
Nature Medicine (2013)
-
AAV-based gene therapy prevents neuropathology and results in normal cognitive development in the hyperargininemic mouse
Gene Therapy (2013)
-
Sustained correction of OTC deficiency in spfash mice using optimized self-complementary AAV2/8 vectors
Gene Therapy (2012)
-
Correction of argininosuccinate synthetase (AS) deficiency in a murine model of citrullinemia with recombinant adenovirus carrying human AS cDNA
Gene Therapy (2000)