Abstract
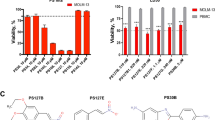
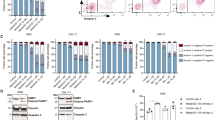
Evasion of apoptosis in pediatric acute lymphoblastic leukemia (ALL) is linked to aberrant expression of inhibitor of apoptosis (IAP) proteins and dysregulated redox homeostasis, rendering leukemic cells vulnerable to redox-targeting therapies. Here we discover that inhibition of antioxidant defenses via glutathione (GSH) depletion by buthionine sulfoximine (BSO) primes ALL cells for apoptosis induced by the Smac mimetic BV6 that antagonizes IAP proteins. Similarly, BSO cooperates with BV6 to induce cell death in patient-derived primary leukemic samples, underscoring the clinical relevance. In contrast, BSO does not sensitize non-malignant lymphohematopoietic cells from healthy donors toward BV6, pointing to some tumor selectivity. Mechanistically, both agents cooperate to stimulate reactive oxygen species (ROS) production, which is required for BSO/BV6-induced cell death, as ROS inhibitors (that is, N-acetylcysteine, MnTBAP, Trolox) significantly rescue cell death. Further, BSO and BV6 cooperate to trigger lipid peroxidation, which is necessary for cell death, as genetic or pharmacological blockage of lipid peroxidation by GSH peroxidase 4 (GPX4) overexpression or α-tocopherol significantly inhibits BSO/BV6-mediated cell death. Consistently, GPX4 knockdown or GPX4 inhibitor RSL3 enhances lipid peroxidation and cell death by BSO/BV6 cotreatment. The discovery of redox regulation of Smac mimetic-induced cell death has important implications for developing rational Smac mimetic-based combination therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pui CH, Relling MV, Downing JR . Acute lymphoblastic leukemia. N Engl J Med 2004; 350: 1535–1548.
Stanulla M, Schrappe M . Treatment of childhood acute lymphoblastic leukemia. Semin Hematol 2009; 46: 52–63.
Fulda S . Tumor resistance to apoptosis. Int J Cancer 2009; 124: 511–515.
Fulda S . Therapeutic opportunities for counteracting apoptosis resistance in childhood leukaemia. Br J Haematol 2009; 145: 441–454.
Fulda S, Debatin KM . Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006; 25: 4798–4811.
Fulda S, Vucic D . Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 2012; 11: 109–124.
Hundsdoerfer P, Dietrich I, Schmelz K, Eckert C, Henze G . XIAP expression is post-transcriptionally upregulated in childhood ALL and is associated with glucocorticoid response in T-cell ALL. Pediatr Blood Cancer 2010; 55: 260–266.
Wuchter C, Richter S, Oltersdorf D, Karawajew L, Ludwig WD, Tamm I . Differences in the expression pattern of apoptosis-related molecules between childhood and adult de novo acute myeloid leukemia. Haematologica 2004; 89: 363–364.
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131: 669–681.
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 2007; 131: 682–693.
Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 2007; 12: 445–456.
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P . Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–1374.
Irwin ME, Rivera-Del Valle N, Chandra J . Redox control of leukemia: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2013; 18: 1349–1383.
Gorrini C, Harris IS, Mak TW . Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013; 12: 931–947.
Yang WS, Stockwell BR . Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 2008; 15: 234–245.
Repetto M, Semprine J, Boveris A . Chemical mechanism, biological implications and analytical determination. In: Catala A (ed). Lipid Peroxidation. InTech Europe, Rijeka, Croatia, 2012, pp 3–30.
Brigelius-Flohe R, Maiorino M . Glutathione peroxidases. Biochim Biophys Acta 2013; 1830: 3289–3303.
Yang WS, Sriramaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014; 156: 317–331.
Friesen C, Kiess Y, Debatin KM . A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ 2004; 11 (Suppl 1): S73–S85.
Chen D, Chan R, Waxman S, Jing Y . Buthionine sulfoximine enhancement of arsenic trioxide-induced apoptosis in leukemia and lymphoma cells is mediated via activation of c-Jun NH2-terminal kinase and up-regulation of death receptors. Cancer Res 2006; 66: 11416–11423.
Trachootham D, Alexandre J, Huang P . Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–591.
Griffith OW . Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem 1982; 257: 13704–13712.
Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci USA 2006; 103: 15038–15043.
Day BJ, Fridovich I, Crapo JD . Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys 1997; 347: 256–262.
Davies MJ, Forni LG, Willson RL . Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem J 1988; 255: 513–522.
Day BJ, Batinic-Haberle I, Crapo JD . Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic Biol Med 1999; 26: 730–736.
Caglikulekci M, Dirlik M, Pata C, Plasse M, Tamer L, Ogetman Z et al. Effect of N-acetylcysteine on blood and tissue lipid peroxidation in lipopolysaccharide-induced obstructive jaundice. J Invest Surg 2006; 19: 175–184.
Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA et al. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal 2010; 12: 1431–1470.
Ni Y, Eng C . Vitamin E protects against lipid peroxidation and rescues tumorigenic phenotypes in cowden/cowden-like patient-derived lymphoblast cells with germline SDHx variants. Clin Cancer Res 2012; 18: 4954–4961.
Naguib YM . A fluorometric method for measurement of peroxyl radical scavenging activities of lipophilic antioxidants. Anal Biochem 1998; 265: 290–298.
Halliwell B, Gutteridge JM . Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984; 219: 1–14.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012; 149: 1060–1072.
Basit F, Humphreys R, Fulda S . RIP1 protein-dependent assembly of a cytosolic cell death complex is required for inhibitor of apoptosis (IAP) inhibitor-mediated sensitization to lexatumumab-induced apoptosis. J Biol Chem 2012; 287: 38767–38777.
Loder S, Fakler M, Schoeneberger H, Cristofanon S, Leibacher J, Vanlangenakker N et al. RIP1 is required for IAP inhibitor-mediated sensitization of childhood acute leukemia cells to chemotherapy-induced apoptosis. Leukemia 2012; 26: 1020–1029.
Abhari BA, Cristofanon S, Kappler R, von Schweinitz D, Humphreys R, Fulda S . RIP1 is required for IAP inhibitor-mediated sensitization for TRAIL-induced apoptosis via a RIP1/FADD/caspase-8 cell death complex. Oncogene 2013; 32: 3263–3273.
Eckhardt I, Roesler S, Fulda S . Identification of DR5 as a critical, NF-kappaB-regulated mediator of Smac-induced apoptosis. Cell Death Dis 2013; 4: e936.
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011; 43: 449–463.
Gomes A, Fernandes E, Lima JL . Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 2005; 65: 45–80.
Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 2012; 52: 1–6.
Franco R, DeHaven WI, Sifre MI, Bortner CD, Cidlowski JA . Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J Biol Chem 2008; 283: 36071–36087.
Franco R, Cidlowski JA . Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 2009; 16: 1303–1314.
Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 2008; 8: 237–248.
Latchoumycandane C, Marathe GK, Zhang R, McIntyre TM . Oxidatively truncated phospholipids are required agents of tumor necrosis factor alpha (TNFalpha)-induced apoptosis. J Biol Chem 2012; 287: 17693–17705.
Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 2012; 36: 215–227.
Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell 2009; 137: 721–735.
Fulda S . Inhibitor of Apoptosis (IAP) proteins in hematological malignancies: molecular mechanisms and therapeutic opportunities. Leukemia 2014; 28: 1414–1422.
Fulda S . Inhibitor of apoptosis proteins in pediatric leukemia: molecular pathways and novel approaches to therapy. Front Oncol 2014; 4: 3.
Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F et al. High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemia. Clin Cancer Res 2004; 10: 3737–3744.
Kearns PR, Pieters R, Rottier MM, Pearson AD, Hall AG . Raised blast glutathione levels are associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood 2001; 97: 393–398.
Maung ZT, Hogarth L, Reid MM, Proctor SJ, Hamilton PJ, Hall AG . Raised intracellular glutathione levels correlate with in vitro resistance to cytotoxic drugs in leukaemic cells from patients with acute lymphoblastic leukemia. Leukemia 1994; 8: 1487–1491.
Fulda S . Molecular pathways: targeting inhibitor of apoptosis proteins in cancer—from molecular mechanism to therapeutic application. Clin Cancer Res 2014; 20: 289–295.
Curtis J, Hedley DW, Minden MD, Moore MA, McCulloch EA . Antileukemic effects of buthionine sulfoximine (BSO) (NSC 326231) in vivo: a pilot study in acute myeloblastic leukemia. In: Büchner T, Hiddemann W, Wörmann B, Schellong G, Ritter J, Creutzig U (eds). Acute Leukemias VIII. Springer: Berlin Heidelberg, Germany, 2001, pp 257–264.
Bailey HH, Ripple G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C et al. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst 1997; 89: 1789–1796.
Fakler M, Loeder S, Vogler M, Schneider K, Jeremias I, Debatin KM et al. Small molecule XIAP inhibitors cooperate with TRAIL to induce apoptosis in childhood acute leukemia cells and overcome Bcl-2-mediated resistance. Blood 2009; 113: 1710–1722.
Gonzalez P, Mader I, Tchoghandjian A, Enzenmuller S, Cristofanon S, Basit F et al. Impairment of lysosomal integrity by B10, a glycosylated derivative of betulinic acid, leads to lysosomal cell death and converts autophagy into a detrimental process. Cell Death Differ 2012; 19: 1337–1346.
Brigelius-Flohe R, Friedrichs B, Maurer S, Schultz M, Streicher R . Interleukin-1-induced nuclear factor kappa B activation is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem J 1997; 328 (Pt 1): 199–203.
Fulda S, Strauss G, Meyer E, Debatin KM . Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells. Blood 2000; 95: 301–308.
Belz K, Schoeneberger H, Wehner S, Weigert A, Bonig H, Klingebiel T et al. Smac mimetic and glucocorticoids synergize to induce apoptosis in childhood ALL by promoting ripoptosome assembly. Blood 2014; 124: 240–250.
Chou TC . Comparison of dose-effect relationships of carcinogens following low-dose chronic exposure and high-dose single injection: an analysis by the median-effect principle. Carcinogenesis 1980; 1: 203–213.
Acknowledgements
We thank D Vucic (Genentech Inc., South San Francisco, CA) for providing BV6, R Brigelius-Flohe for providing the GPX4 vector, T Klingebiel and S Wehner for providing ALL samples, J Leibacher for providing MSCs, PF Angeli and M Conrad for helpful discussions and C Hugenberg for expert secretarial assistance. This work has been partially supported by grants from the Deutsche Forschungsgemeinschaft and IUAP VII (to SF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Schoeneberger, H., Belz, K., Schenk, B. et al. Impairment of antioxidant defense via glutathione depletion sensitizes acute lymphoblastic leukemia cells for Smac mimetic-induced cell death. Oncogene 34, 4032–4043 (2015). https://doi.org/10.1038/onc.2014.338
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.338
This article is cited by
-
The Role of Redox Status Changes in Dexamethasone-Induced Apoptosis in Jurkat Tumor Cells
Bulletin of Experimental Biology and Medicine (2022)
-
Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy
Cell Death & Disease (2020)
-
Reactive oxygen species in haematopoiesis: leukaemic cells take a walk on the wild side
Journal of Experimental & Clinical Cancer Research (2018)
-
Emerging roles for lipids in non-apoptotic cell death
Cell Death & Differentiation (2016)
-
Mechanisms of ferroptosis
Cellular and Molecular Life Sciences (2016)