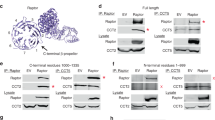
Abstract
Growth factor signaling coupled to activation of the phosphatidylinositol-3-OH kinase (PI3K)/Akt pathway plays a crucial role in the regulation of cell proliferation and survival. The key regulatory kinase of Akt has been identified as mammalian target of rapamycin complex 2 (mTORC2), which functions as the PI3K-dependent Ser-473 kinase of Akt. This kinase complex is assembled by mTOR and its essential components rictor, Sin1 and mLST8. The recent genetic screening study in Caenorhabditis elegans has linked a specific point mutation of rictor to an elevated storage of fatty acids that resembles the rictor deficiency phenotype. In our study, we show that in mammalian cells the analogous single rictor point mutation (G934E) prevents the binding of rictor to Sin1 and the assembly of mTORC2, but this mutation does not interfere with the binding of the rictor-interacting protein Protor. A substitution of the rictor Gly-934 residue to a charged amino acid prevents formation of the rictor/Sin1 heterodimer. The cells expressing the rictor G934E mutant remain deficient in the mTORC2 signaling, as detected by the reduced phosphorylation of Akt on Ser-473 and a low cell proliferation rate. Thus, although a full length of rictor is required to interact with its binding partner Sin1, a single amino acid of rictor Gly-934 controls its interaction with Sin1 and assembly of mTORC2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB et al. (1997). Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7: 261–269.
Bayascas JR . (2008). Dissecting the role of the 3-phosphoinositide-dependent protein kinase-1 (PDK1) signalling pathways. Cell Cycle 7: 2978–2982.
Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D et al. (1998). Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17: 313–325.
Bozulic L, Surucu B, Hynx D, Hemmings BA . (2008). PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell 30: 203–213.
Cantley LC . (2002). The phosphoinositide 3-kinase pathway. Science 296: 1655–1657.
Chen C-H, Sarbassov DD . (2011). The mTOR kinase activity determines functional activity and integrity of mTORC2. J Biol Chem (resubmitted).
Chen CH, Shaikenov T, Peterson TR, Aimbetov R, Bissenbaev AK, Lee SW et al. (2011). ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci Signal 4: ra10.
Citri A, Yarden Y . (2006). EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7: 505–516.
Cordes MH, Davidson AR, Sauer RT . (1996). Sequence space, folding and protein design. Curr Opin Struct Biol 6: 3–10.
Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA et al. (2006). mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 16: 1865–1870.
Gross JM, Yee D . (2003). The type-1 insulin-like growth factor receptor tyrosine kinase and breast cancer: biology and therapeutic relevance. Cancer Metastasis Rev 22: 327–336.
Guertin DA, Sabatini DM . (2005). An expanding role for mTOR in cancer. Trends Mol Med 11: 353–361.
Guertin DA, Sabatini DM . (2007). Defining the role of mTOR in cancer. Cancer Cell 12: 9–22.
Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY et al. (2006). SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137.
Jones KT, Greer ER, Pearce D, Ashrafi K . (2009). Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol 7: e60.
Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M et al. (2007). Identification of Protor as a novel rictor-binding component of mTOR complex-2. Biochem J 405: 513–522.
Pearce LR, Komander D, Alessi DR . (2010). The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22.
Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR . (2011). Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J 436: 169–179.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H et al. (2004). Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302.
Sarbassov DD, Ali SM, Sabatini DM . (2005a). Growing roles for the mTOR pathway. Curr Opin Cell Biol 17: 596–603.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM . (2005b). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101.
Schlessinger J, Lemmon MA . (2003). SH2 and PTB domains in tyrosine kinase signaling. Sci STKE 2003: RE12.
Shaw RJ, Cantley LC . (2006). Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441: 424–430.
Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G . (2009). Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev 23: 496–511.
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB et al. (1998). Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279: 710–714.
Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI et al. (2007). PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem 282: 25604–25612.
Zhang X, Yee D . (2000). Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res 2: 170–175.
Acknowledgements
This study was supported by the MD Anderson Trust Fellow Fund and NIH grant CA133522 (DDS). R Aimbetov and O Bulgakova have been partially supported by the Ph.D. training grants from Kazakhstan. We are thankful to our laboratory member Dr Tattym Shaikenov for providing the Akt substrate. We are also thankful to Dr Dario Alessi (University of Dundee, Dundee, England) for providing the Protor 1α expression plasmid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Aimbetov, R., Chen, CH., Bulgakova, O. et al. Integrity of mTORC2 is dependent on the rictor Gly-934 site. Oncogene 31, 2115–2120 (2012). https://doi.org/10.1038/onc.2011.404
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.404
Keywords
This article is cited by
-
UBXN2A suppresses the Rictor-mTORC2 signaling pathway, an established tumorigenic pathway in human colorectal cancer
Oncogene (2023)
-
Target of Rapamycin Complex 2 regulates cell growth via Myc in Drosophila
Scientific Reports (2015)
-
The evolution of the TOR pathway and its role in cancer
Oncogene (2013)
-
Rictor regulates cell migration by suppressing RhoGDI2
Oncogene (2013)