Abstract
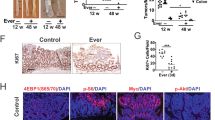
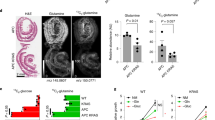
The adenomatous polyposis coli (APC) gene is mutated in familial adenomatous polyposis. Mice with a heterozygous APCMin mutation develop multiple intestinal neoplasia (Min) leading to premature death. Early in colorectal carcinogenesis, APCMin/+ mice show enhanced Akt-mammalian target of rapamycin (mTOR) signaling, which is paralleled by upregulation of oncogenic K+ channels. In this study, we tested the effect of mTOR inhibition with rapamycin on tumor formation in APCMin/+ mice and evaluated ion channel regulation. We found that continuous long-term rapamycin treatment of APCMin/+ mice dramatically inhibits intestinal neoplasia. Moreover, although untreated APCMin/+ mice lose weight, experience intestinal bleeding and succumb to multiple neoplasia by 22.3±1.4 weeks of age, mice treated with rapamycin maintain stable weight and survive long term (39.6±3.4 weeks), with more than 30% surviving >1 year. Impressively, abnormalities in colonic electrolyte transport typical for APCMin/+ mice are abolished, along with the suppression of epithelial Na+ channel (ENaC) and oncogenic K+ ion channels BK, Elk1 and Erg1, both functionally and at mRNA levels. These results show that continuous prophylaxis by rapamycin markedly inhibits the development of APC mutation-related polyposis, and suggest a novel contributing mechanism of action through the blockade of intestinal oncogenic ion channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aoki K, Taketo MM . (2007). Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci 120: 3327–3335.
Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM . (2003). Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Delta716 Cdx2+/− compound mutant mice. Nat Genet 35: 323–330.
Dihlmann S, Kloor M, Fallsehr C, von Knebel Doeberitz M . (2005). Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis 26: 1503–1512.
Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K, Sansom O et al. (2007). Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol 176: 183–195.
Fingar DC, Salama S, Tsou C, Harlow E, Blenis J . (2002). Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16: 1472–1487.
Fodde R, Edelmann W, Yang K, van LC, Carlson C, Renault B et al. (1994). A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 91: 8969–8973.
Fodde R, Smits R, Clevers H . (2001). APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 1: 55–67.
Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM . (2008). Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA 105: 13544–13549.
Gaber AO, Kahan BD, Van BC, Schulman SL, Scarola J, Neylan JF . (2008). Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation 86: 1187–1195.
Guba M, Koehl E, Neppl E, Doenecke A, Steinbauer M, Schlitt J et al. (2005). Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transpl Int 18: 89–94.
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X et al. (2006). TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968.
Koehl GE, Gaumann A, Zuelke C, Hoehn A, Hofstaedter F, Schlitt HJ et al. (2006). Development of de novo cancer in p53 knock-out mice is dependent on the type of long-term immunosuppression used. Transplantation 82: 741–748.
Koehl GE, Schlitt HJ, Geissler EK . (2005). Rapamycin and tumor growth: mechanisms behind its anticancer activity. Transplant Rev 19: 20–31.
Kunzelmann K . (2005). Ion channels and cancer. J Membr Biol 205: 159–173.
Moran AE, Hunt DH, Javid SH, Redston M, Carothers AM, Bertagnolli MM . (2004). Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J Biol Chem 279: 43261–43272.
Moser AR, Luongo C, Gould KA, McNeley MK, Shoemaker AR, Dove WF . (1995). ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur J Cancer 31A: 1061–1064.
Otulakowski G, Duan W, Gandhi S, O'Brodovich H . (2007). Steroid and oxygen effects on eIF4F complex, mTOR, and ENaC translation in fetal lung epithelia. Am J Respir Cell Mol Biol 37: 457–466.
Ousingsawat J, Spitzner M, Puntheeranurak S, Terracciano L, Tornillo L, Bubendorf L et al. (2007). Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res 13: 824–831.
Ousingsawat J, Spitzner M, Schreiber R, Kunzelmann K . (2008). Upregulation of colonic ion channels in APC (Min/+) mice. Pflugers Arch 456: 847–855.
Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stuhmer W . (2005). Role of voltage-gated potassium channels in cancer. J Membr Biol 205: 115–124.
Pardo LA, del CD, Sanchez A, Alves F, Bruggemann A, Beckh S et al. (1999). Oncogenic potential of EAG K(+) channels. EMBO J 18: 5540–5547.
Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V et al. (2007). VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood 110: 1238–1250.
Puntheeranurak S, Schreiber R, Spitzner M, Ousingsawat J, Krishnamra N, Kunzelmann K . (2007). Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem 19: 77–88.
Schreiber R . (2005). Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol 205: 129–137.
Spitzner M, Martins JR, Soria RB, Ousingsawat J, Scheidt K, Schreiber R et al. (2008). Eag1 and Bestrophin 1 are up-regulated in fast-growing colonic cancer cells. J Biol Chem 283: 7421–7428.
Spitzner M, Ousingsawat J, Scheidt K, Kunzelmann K, Schreiber R . (2007). Voltage-gated K+ channels support proliferation of colonic carcinoma cells. FASEB J 21: 35–44.
Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C et al. (1992). Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256: 668–670.
Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV . (2006). Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res 66: 7370–7377.
Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI et al. (1999). Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 285: 1929–1931.
Wonderlin WF, Strobl JS . (1996). Potassium channels, proliferation and G1 progression. J Membr Biol 154: 91–107.
Yao X, Kwan HY . (1999). Activity of voltage-gated K+ channels is associated with cell proliferaton and Ca2+ influx in carcinoma cells of colon cancer. Life Sci 65: 55–62.
Zhang Y, Wang H, Wang J, Han H, Nattel S, Wang Z . (2003). Normal function of HERG K+ channels expressed in HEK293 cells requires basal protein kinase B activity. FEBS Lett 534: 125–132.
Acknowledgements
We thank Anna Hoehn, Astrid Schwend and Ernestine Tartler for their excellent technical assistance. This study was supported by grants from the Deutsche Forschungsgemeinschaft to RS and KK (SCHR 752/2–2, SFB699, KU 756/8–2), the Wilhelm Sander-Stiftung (2005.063.1; to KK) and the Roche Organ Transplant Research Foundation (to EKG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Koehl, G., Spitzner, M., Ousingsawat, J. et al. Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APCMin/+ mice. Oncogene 29, 1553–1560 (2010). https://doi.org/10.1038/onc.2009.435
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.435
Keywords
This article is cited by
-
Targeting the biology of aging with mTOR inhibitors
Nature Aging (2023)
-
Effect of rapamycin on aging and age-related diseases—past and future
GeroScience (2021)
-
A Review of Compounds for Prevention of Colorectal Cancer
Current Pharmacology Reports (2017)
-
Wnt Signaling in Normal and Malignant Stem Cells
Current Stem Cell Reports (2016)
-
Rectal forceps biopsy procedure in cystic fibrosis: technical aspects and patients perspective for clinical trials feasibility
BMC Gastroenterology (2013)