Abstract
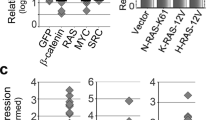
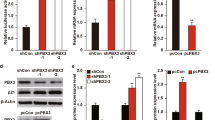
There is emerging evidence that the oncogenic potential of hdm2 (human and/or murine double minute-2 protein) stems not only from its ability to counteract tumor suppressor p53 but also from its less understood p53-independent functions. Surprisingly, little is known about the role and regulation of hdm2 in pancreatic tumors, a large proportion (50–75%) of which contain mutant p53. In this study, we determined that hdm2 was expressed in a Ras-signaling-dependent manner in various pancreatic cancer cell lines. As p53 was mutated and inactive in these cells, the expression of hdm2 was seemingly redundant. Indeed, the proliferation and survival of cell lines such as Panc-1 and Panc-28 could be inhibited by PRIMA-1 (mutant p53 activator) but not by Nutlin-3 (inhibitor of the hdm2–p53 interaction). Unexpectedly, however, the proliferation of both cell lines was strongly inhibited by hdm2-specific RNAi. Our data also revealed cyclin D1, c-Jun and c-Myc to be novel targets of hdm2 and suggested that they might mediate hdm2's role in cellular proliferation and/or survival. We conclude from our results that hdm2 is expressed in pancreatic cancer cells as a result of activated Ras signaling, and that it regulates cellular proliferation and the expression of three novel target genes by p53-independent mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- hdm2:
-
human and/or murine double minute-2 protein
- MEK:
-
mitogen and extracellular signal-regulated protein kinase
- p53:
-
tumor suppressor p53
References
Agrawal S, Kandimalla ER, Yu D, Hollister BA, Chen SF, Dexter DL et al. (2001). Potentiation of antitumor activity of irinotecan by chemically modified oligonucleotides. Int J Oncol 18: 1061–1069.
Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK . (2007). Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene 26: 3473–3481.
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA . (2004). The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene 23: 8571–8580.
Bai J, Cederbaum AI . (2006). Cycloheximide protects HepG2 cells from serum withdrawal-induced apoptosis by decreasing p53 and phosphorylated p53 levels. J Pharmacol Exp Ther 319: 1435–1443.
Bartel F, Taubert H, Harris LC . (2002). Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer Cell 2: 9–15.
Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC et al. (2004). A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119: 591–602.
Brummelkamp TR, Bernards R, Agami R . (2002). Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2: 243–247.
Brunner TB, Cengel KA, Hahn SM, Wu J, Fraker DL, McKenna WG et al. (2005). Pancreatic cancer cell radiation survival and prenyltransferase inhibition: the role of K-Ras. Cancer Res 65: 8433–8441.
Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P et al. (2002). Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 8: 282–288.
Carroll VA, Ashcroft M . (2008). Regulation of angiogenic factors by HDM2 in renal cell carcinoma. Cancer Res 68: 545–552.
Chipuk JE, Maurer U, Green DR, Schuler M . (2003). Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell 4: 371–381.
Daujat S, Neel H, Piette J . (2001). MDM2: life without p53. Trends Genet 17: 459–464.
Deb SP . (2003). Cell cycle regulatory functions of the human oncoprotein MDM2. Mol Cancer Res 1: 1009–1016.
Ebert M, Yokoyama M, Kobrin MS, Friess H, Buchler MW, Korc M . (1994). Increased MDM2 expression and immunoreactivity in human pancreatic ductal adenocarcinoma. Int J Oncol 5: 1279–1284.
Frazier MW, He X, Wang J, Gu Z, Cleveland JL, Zambetti GP . (1998). Activation of c-myc gene expression by tumor-derived p53 mutants requires a discrete C-terminal domain. Mol Cell Biol 18: 3735–3743.
Freedman DA, Wu L, Levine AJ . (1999). Functions of the MDM2 oncoprotein. Cell Mol Life Sci 55: 96–107.
Fridman JS, Hernando E, Hemann MT, de Stanchina E, Cordon-Cardo C, Lowe SW . (2003). Tumor promotion by Mdm2 splice variants unable to bind p53. Cancer Res 63: 5703–5706.
Ganguli G, Wasylyk B . (2003). p53-independent functions of MDM2. Mol Cancer Res 1: 1027–1035.
Harris LC . (2005). MDM2 splice variants and their therapeutic implications. Curr Cancer Drug Targets 5: 21–26.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA . (2006). Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20: 1218–1249.
Ho JS, Ma W, Mao DY, Benchimol S . (2005). p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol 25: 7423–7431.
Iwakuma T, Lozano G . (2003). MDM2, an introduction. Mol Cancer Res 1: 993–1000.
Jackson P, Ow K, Yardley G, Delprado W, Quinn DI, Yang JL et al. (2003). Downregulation of KAI1 mRNA in localised prostate cancer and its bony metastases does not correlate with p53 overexpression. Prostate Cancer Prostatic Dis 6: 174–181.
Jones SN, Hancock AR, Vogel H, Donehower LA, Bradley A . (1998). Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci USA 95: 15608–15612.
Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M et al. (2005). A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 123: 833–847.
Konnikova L, Simeone MC, Kruger MM, Kotecki M, Cochran BH . (2005). Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res 65: 6516–6520.
Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG . (2007). Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J Biol Chem 282: 4102–4112.
LaRusch GA, Jackson MW, Dunbar JD, Warren RS, Donner DB, Mayo LD . (2007). Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1alpha and Hdm2. Cancer Res 67: 450–454.
Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB . (2006). Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci USA 103: 17390–17395.
Levav-Cohen Y, Haupt S, Haupt Y . (2005). Mdm2 in growth signaling and cancer. Growth Factors 23: 183–192.
Lundgren K, Montes de Oca Luna R, McNeill YB, Emerick EP, Spencer B, Barfield CR et al. (1997). Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev 11: 714–725.
Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M et al. (2005). IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res 65: 7573–7579.
Ouyang H, Mou L, Luk C, Liu N, Karaskova J, Squire J et al. (2000). Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol 157: 1623–1631.
Peng Y, Chen L, Li C, Lu W, Chen J . (2001). Inhibition of MDM2 by hsp90 contributes to mutant p53 stabilization. J Biol Chem 276: 40583–40590.
Phelps M, Darley M, Primrose JN, Blaydes JP . (2003). p53-independent activation of the hdm2-P2 promoter through multiple transcription factor response elements results in elevated hdm2 expression in estrogen receptor alpha-positive breast cancer cells. Cancer Res 63: 2616–2623.
Phelps M, Phillips A, Darley M, Blaydes JP . (2005). MEK-ERK signaling controls Hdm2 oncoprotein expression by regulating hdm2 mRNA export to the cytoplasm. J Biol Chem 280: 16651–16658.
Phillips A, Jones CJ, Blaydes JP . (2006). The mechanisms of regulation of Hdm2 protein level by serum growth factors. FEBS Lett 580: 300–304.
Prives C, Hall PA . (1999). The p53 pathway. J Pathol 187: 112–126.
Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T et al. (2000). Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103: 321–330.
Rocha S, Martin AM, Meek DW, Perkins ND . (2003). p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol Cell Biol 23: 4713–4727.
Ruggeri BA, Huang L, Berger D, Chang H, Klein-Szanto AJ, Goodrow T et al. (1997). Molecular pathology of primary and metastatic ductal pancreatic lesions: analyses of mutations and expression of the p53, mdm-2, and p21/WAF-1 genes in sporadic and familial lesions. Cancer 79: 700–716.
Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle-Steinlein U, Tian J et al. (1999). Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev 13: 607–619.
Shaulian E, Resnitzky D, Shifman O, Blandino G, Amsterdam A, Yayon A et al. (1997). Induction of Mdm2 and enhancement of cell survival by bFGF. Oncogene 15: 2717–2725.
Shmueli A, Oren M . (2007). Mdm2: p53's lifesaver? Mol Cell 25: 794–796.
Sigalas I, Calvert AH, Anderson JJ, Neal DE, Lunec J . (1996). Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: transforming ability and frequent detection in human cancer. Nat Med 2: 912–917.
Steinman HA, Burstein E, Lengner C, Gosselin J, Pihan G, Duckett CS et al. (2004). An alternative splice form of Mdm2 induces p53-independent cell growth and tumorigenesis. J Biol Chem 279: 4877–4886.
Sun P, Dong P, Dai K, Hannon GJ, Beach D . (1998). p53-independent role of MDM2 in TGF-beta1 resistance. Science 282: 2270–2272.
van Triest M, de Rooij J, Bos JL . (2001). Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol 333: 343–348.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848.
Walker SR, Nelson EA, Frank DA . (2007). STAT5 represses BCL6 expression by binding to a regulatory region frequently mutated in lymphomas. Oncogene 26: 224–233.
Weisz B, Giehl K, Gana-Weisz M, Egozi Y, Ben-Baruch G, Marciano D et al. (1999). A new functional Ras antagonist inhibits human pancreatic tumor growth in nude mice. Oncogene 18: 2579–2588.
Zhang Z, Wang H, Li M, Rayburn ER, Agrawal S, Zhang R . (2005). Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway. Oncogene 24: 7238–7247.
Zhang Z, Wang H, Prasad G, Li M, Yu D, Bonner JA et al. (2004). Radiosensitization by antisense anti-MDM2 mixed-backbone oligonucleotide in in vitro and in vivo human cancer models. Clin Cancer Res 10: 1263–1273.
Acknowledgements
We are grateful to Drs Frank McCormick and Martin McMahon (University of California, San Francisco, CA, USA) for the hdm2 P2 promoter reporter constructs, Johannes Bos (Utrecht University) for the Raf1 GST-RBD plasmid and Gigi Lozano (M.D. Anderson Cancer Center, Houston, TX, USA) for the p53 and pCMV-hdm2 expression vectors. We also thank Pfizer (CI-1040), Dr Bryant Darnay (pMX-BGD vector) and Dr F-X Claret (pSRα-c-Jun) for other reagents; Dr Baoan Ji for assistance with the qPCR assays and Beth L Notzon and Angelique L Geehan (M.D. Anderson Cancer Center) for editorial assistance. This work was supported by the Lustgarten Foundation for Pancreatic Cancer Research (SAGR), the Topfer Fund for Pancreatic Cancer Research (SAGR), the University Cancer Foundation at The University of Texas M.D. Anderson Cancer Center (SAGR), NCI SPORE CA101936 in Pancreatic Cancer (JLA) and NCI Grant CA016672 (DNA sequencing and media preparation facilities, The University of Texas M.D. Anderson Cancer Center).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Sui, X., Shin, S., Zhang, R. et al. Hdm2 is regulated by K-Ras and mediates p53-independent functions in pancreatic cancer cells. Oncogene 28, 709–720 (2009). https://doi.org/10.1038/onc.2008.423
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.423
Keywords
This article is cited by
-
HDAC1 and HDAC2 integrate the expression of p53 mutants in pancreatic cancer
Oncogene (2017)
-
Cooperation among Numb, MDM2 and p53 in the development and progression of pancreatic cancer
Cell and Tissue Research (2013)
-
Mutated genes in pancreatic cancer
Chinese Journal of Cancer Research (2010)