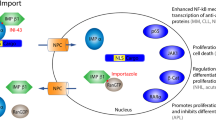
Abstract
Previous studies have described one nuclear localization signal (NLSI) in p53 and speculated on two additional sites termed NLSII and NLSIII. Drug-resistant KB cells selected with cisplatin or oxaliplatin were found to have increased p53 levels and in oxaliplatin-selected cells, a larger p53 predominantly in the cytoplasm. In oxaliplatin-selected cells a single nucleotide deletion in the sequence-encoding amino acid 382, part of NLSIII, resulted in a frame shift and a 420 amino acid protein (p53420). We investigated explanations for the cytoplasmic sequestration of p53420 while assessing the role, if any, of NLSII and NLSIII in p53 nuclear import. We found that neither NLSII nor NLSIII are essential for p53 nuclear localization. Furthermore, we confirmed p53420 is able to tetramerize, transactivate a p21 promoter, bind dynein and that the reduced nuclear accumulation is not a consequence of increased p53 nuclear export. However, the association of p53420 with importin-β, essential for nuclear import, was significantly impaired. We conclude that despite sequence similarity to consensus NLSs neither NLSII nor NLSIII have roles in p53 nuclear transport. We also identified impaired association with importin as a novel mechanism of p53 cytoplasmic sequestration that impairs nuclear transport rendering cells functionally deficient in p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Becker K, Marchenko ND, Maurice M, Moll UM . (2007). Hyperubiquitylation of wild-type p53 contributes to cytoplasmic sequestration in neuroblastoma. Cell Death Differ 14: 1350–1360.
Bosari S, Viale G, Bossi P, Maggioni M, Coggi G, Murray JJ et al. (1994). Cytoplasmic accumulation of p53 protein: an independent prognostic indicator in colorectal adenocarcinomas. J Natl Cancer Inst 86: 681–687.
Bosari S, Viale G, Roncalli M, Graziani D, Borsani G, Lee AK et al. (1995). p53 gene mutations, p53 protein accumulation and compartmentalization in colorectal adenocarcinoma. Am J Pathol 147: 790–798.
Bristow RG, Peacock J, Jang A, Kim J, Hill RP, Benchimol S . (2003). Resistance to DNA-damaging agents is discordant from experimental metastatic capacity in MEF ras-transformants-expressing gain of function MTp53. Oncogene 22: 2960–2966.
Dang CV, Lee WM . (1989). Nuclear and nucleolar targeting sequences of c-erb-A, c-myb, N-myc, p53, HSP70, and HIV tat proteins. J Biol Chem 264: 18019–18023.
Dingwall C, Dilworth SM, Black SJ, Kearsey SE, Cox LS, Laskey RA . (1987). Nucleoplasmin cDNA sequence reveals polyglutamic acid tracts and a cluster of sequences homologous to putative nuclear localization signals. EMBO J 6: 69–74.
el-Deiry WS . (1998). Regulation of p53 downstream genes. Semin Cancer Biol 8: 345–357.
Freedman DA, Levine AJ . (1998). Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol 18: 7288–7293.
Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T . (2000). p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol 2: 709–717.
Hollstein M, Sidransky D, Vogelstein B, Harris CC . (1991). p53 mutations in human cancers. Science 253: 49–53.
Imamura J, Bartram CR, Berthold F, Harms D, Nakamura H, Koeffler HP . (1993). Mutation of the p53 gene in neuroblastoma and its relationship with N-myc amplification. Cancer Res 53: 4053–4058.
Kim IS, Kim DH, Han SM, Chin MU, Nam HJ, Cho HP et al. (2000). Truncated form of importin alpha identified in breast cancer cell inhibits nuclear import of p53. J Biol Chem 275: 23139–23145.
Lane DP . (1992). Cancer. p53, guardian of the genome. Nature 358: 15–16.
Levine AJ, Momand J, Finlay CA . (1991). The p53 tumour suppressor gene. Nature 351: 453–456.
Li Q, Falsey RR, Gaitonde S, Sotello V, Kislin K, Martinez JD . (2007). Genetic analysis of p53 nuclear importation. Oncogene 26: 7885–7893.
Liang SH, Clarke MF . (1999). A bipartite nuclear localization signal is required for p53 nuclear import regulated by a carboxyl-terminal domain. J Biol Chem 274: 32699–32703.
Lilling G, Nordenberg J, Rotter V, Goldfinger N, Peller S, Sidi Y . (2002). Altered subcellular localization of p53 in estrogen-dependent and estrogen-independent breast cancer cells. Cancer Invest 20: 509–517.
Moll UM, LaQuaglia M, Benard J, Riou G . (1995). Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc Natl Acad Sci USA 92: 4407–4411.
O′Brate A, Giannakakou P . (2003). The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist Updat 6: 313–322.
Oggionni M, Pilotti S, Suardi S, Ditto A, Luoni C, Mariani L et al. (2005). p53 Gene status and response to topotecan-containing chemotherapy in advanced ovarian carcinoma. Oncology 69: 154–158.
Ottaggio L, Bozzo S, Moro F, Sparks A, Campomenosi P, Miele M et al. (2000). Defective nuclear localization of p53 protein in a Chinese hamster cell line is associated with the formation of stable cytoplasmic protein multimers in cells with gene amplification. Carcinogenesis 21: 1631–1638.
Qu L, Huang S, Baltzis D, Rivas-Estilla AM, Pluquet O, Hatzoglou M et al. (2004). Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev 18: 261–277.
Riddick G, Macara IG . (2005). A systems analysis of importin-\{alpha\}-\{beta\} mediated nuclear protein import. J Cell Biol 168: 1027–1038.
Roth J, Dobbelstein M, Freedman DA, Shenk T, Levine AJ . (1998). Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J 17: 554–564.
Ryan KM, Phillips AC, Vousden KH . (2001). Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol 13: 332–337.
Scata KA, El-Deiry WS . (2007). p53, BRCA1 and breast Cancer chemoresistance. Adv Exp Med Biol 608: 70–86.
Sembritzki O, Hagel C, Lamszus K, Deppert W, Bohn W . (2002). Cytoplasmic localization of wild-type p53 in glioblastomas correlates with expression of vimentin and glial fibrillary acidic protein. Neuro Oncol 4: 171–178.
Shaulsky G, Goldfinger N, Ben-Ze′ev A, Rotter V . (1990). Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol 10: 6565–6577.
Shaulsky G, Goldfinger N, Tosky MS, Levine AJ, Rotter V . (1991). Nuclear localization is essential for the activity of p53 protein. Oncogene 6: 2055–2065.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112.
Smith AE, Kalderon D, Roberts BL, Colledge WH, Edge M, Gillett P et al. (1985). The nuclear location signal. Proc R Soc Lond B Biol Sci 226: 43–58.
Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM . (1999). A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J 18: 1660–1672.
Sun XF, Carstensen JM, Zhang H, Stal O, Wingren S, Hatschek T et al. (1992). Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet 340: 1369–1373.
Trostel SY, Sackett DL, Fojo T . (2006). Oligomerization of p53 precedes its association with dynein and nuclear accumulation. Cell Cycle 5: 2253–2259.
Utama B, Shen YH, Mitchell BM, Makagiansar IT, Gan Y, Muthuswamy R et al. (2006). Mechanisms for human cytomegalovirus-induced cytoplasmic p53 sequestration in endothelial cells. J Cell Sci 119: 2457–2467.
Weiss J, Heine M, Korner B, Pilch H, Jung EG . (1995). Expression of p53 protein in malignant melanoma: clinicopathological and prognostic implications. Br J Dermatol 133: 23–31.
Woods DB, Vousden KH . (2001). Regulation of p53 function. Exp Cell Res 264: 56–66.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Komlodi-Pasztor, E., Trostel, S., Sackett, D. et al. Impaired p53 binding to importin: a novel mechanism of cytoplasmic sequestration identified in oxaliplatin-resistant cells. Oncogene 28, 3111–3120 (2009). https://doi.org/10.1038/onc.2009.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.166
Keywords
This article is cited by
-
G-actin guides p53 nuclear transport: potential contribution of monomeric actin in altered localization of mutant p53
Scientific Reports (2016)
-
Heterozygous p53V172F mutation in cisplatin-resistant human tumor cells promotes MDM4 recruitment and decreases stability and transactivity of p53
Oncogene (2016)
-
RETRACTED ARTICLE: Up-Regulation of KPNB1 Involves in Neuronal Apoptosis Following Intracerebral Hemorrhage in Adult Rats
Neurochemical Research (2015)
-
TIP30 Directly Binds p53 Tumor Suppressor Protein In Vitro
Molecules and Cells (2012)