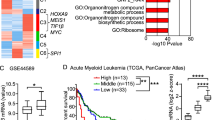
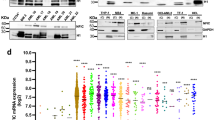
Abstract
Promyelocytic leukemia (PML) protein is a tumor suppressor with complicated action mechanisms not yet fully understood. In this study, we found that the nuclear factor of activated T cell (NFAT) is an unexpected partner of PML: PML specifically enhanced the transcription activation of NFAT. In PML-null mouse embryonic fibroblasts, no transcription activity of NFAT could be detected. There was a selective requirement of PML isoform in NFAT activation: PML-I and PML-VI, but not PML-IV, increased NFAT transactivation. PML specifically promoted the expression of many, but not all, NFAT-targeted genes. We found a specific binding of PML to NFATc. The interaction of PML with NFATc in vivo was further confirmed by chromatin immunoprecipitation and DNA affinity precipitation assay analysis. The unexpected coupling of PML with NFAT reveals a novel mechanism underlying the diverse physiological functions of PML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Avots A, Buttmann M, Chuvpilo S, Escher C, Smola U, Bannister AJ et al. (1999). CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity 10: 515–524.
Bischof O, Kirsh O, Pearson M, Itahana K, Pelicci PG, Dejean A . (2002). Deconstructing PML-induced premature senescence. EMBO J 21: 3358–3369.
Bruno S, Ghiotto F, Fais F, Fagioli M, Luzi L, Pelicci PG et al. (2003). The PML gene is not involved in the regulation of MHC class I expression in human cell lines. Blood 101: 3514–3519.
Buschbeck M, Uribesalgo I, Ledl A, Gutierrez A, Minucci S, Muller S et al. (2007). PML4 induces differentiation by Myc destabilization. Oncogene 26: 3415–3422.
Cairo S, De Falco F, Pizzo M, Salomoni P, Pandolfi PP, Meroni G . (2005). PML interacts with Myc, and Myc target gene expression is altered in PML-null fibroblasts. Oncogene 24: 2195–2203.
Condemine W, Takahashi Y, Zhu J, Puvion-Dutilleul F, Guegan S, Janin A et al. (2006). Characterization of endogenous human promyelocytic leukemia isoforms. Cancer Res 66: 6192–6198.
Crabtree GR, Olson EN . (2002). NF-AT signaling: choreographing the social lives of cells. Cell 109: S67–S79.
Crowder C, Dahle O, Davis RE, Gabrielsen OS, Rudikoff S . (2005). PML mediates IFN-α-induced apoptosis in myeloma by regulating TRAIL induction. Blood 105: 1280–1287.
Dellaire G, Bazett-Jones DP . (2004). PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays 26: 963–977.
Doucas V, Tini M, Egan DA, Evans RM . (1999). Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc Natl Acad Sci USA 96: 2627–2632.
Fogal V, Gostissa M, Sandy P, Zacchi P, Sternsdorf T, Jensen K et al. (2000). Regulation of p53 activity in nuclear bodies by a specific PML isoform. EMBO J 19: 6185–6195.
Garcia-Rodriguez C, Rao A . (1998). Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein. J Exp Med 187: 2031–2036.
Goddard AD, Yuan JQ, Fairbarin L, Dexter M, Borrow J, Kozak C et al. (1995). Cloning of the murine homolog of the leukemia-associated PML gene. Mamm Genome 6: 732–737.
Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W et al. (2000). The function of PML in p53-dependent apoptosis. Nat Cell Biol 2: 730–736.
Ho HY, Lee HH, Lai MZ . (1997). Overexpression of mitogen-activated protein kinase kinase kinase reversed cAMP inhibition of NF-κB in T cells. Eur J Immunol 27: 222–226.
Hogan PG, Chen L, Nardone J, Rao A . (2003). Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232.
Ishov AM, Vladimirova OV, Maul GG . (2004). Heterochromatin and ND10 are cell-cycle regulated and phosphorylation-dependent alternate nuclear sites of the transcription repressor Daxx and SWI/SNF protein ATRX. J Cell Sci 117: 3807–3820.
Jensen K, Shiels C, Freemont PS . (2001). PML protein isoforms and the RBCC/TRIM motif. Oncogene 20: 7223–7233.
Latinis KM, Norian LA, Eliason SL, Koretzky GA . (1997). Two NFAT transcription factor binding sites participate in the regulation of CD95 (Fas) ligand expression in activated human T cells. J Biol Chem 272: 31427–31434.
Li H, Leo C, Zhu J, Wu X, O'Neil J, Park EJ et al. (2000). Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol Cell Biol 20: 1784–1796.
Lin DY, Fang HI, Ma AH, Huang YS, Pu YS, Jenster G et al. (2004). Negative modulation of androgen receptor transcriptional activity by Daxx. Mol Cell Biol 24: 10529–10541.
Lin DY, Lai MZ, Ann DK, Shih HM . (2003). Promyelocytic leukemia protein (PML) functions as a glucocorticoid receptor co-activator by sequestering Daxx to the PML oncogenic domains (PODs) to enhance its transactivation potential. J Biol Chem 278: 15958–15965.
Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A . (2002). Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109: 719–731.
Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M et al. (2003). Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115: 751–763.
Möller A, Sirma H, Hofmann TG, Rueffer S, Klimczak E, Dröge W et al. (2003). PML is required for homeodomain-interacting protein kinase 2 (HIPK2)-mediated p53 phosphorylation and cell cycle arrest but is dispensable for the formation of HIPK domains. Cancer Res 63: 4310–4314.
Perkins ND, Felzien LK, Betts JC, Leung K, Beach DH, Nabel GJ . (1997). Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science 275: 523–527.
Salomoni P, Bernardi R, Bergmann S, Changou A, Tuttle S, Pandolfi PP . (2005). The promyelocytic leukemia protein PML regulates c-Jun function in response to DNA damage. Blood 105: 3686–3690.
Salomoni P, Pandolfi PP . (2002). The role of PML in tumor suppression. Cell 108: 165–170.
Tsai EY, Yie J, Thanos D, Goldfeld AE . (1996). Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol 16: 5232–5244.
von Mikecz A, Zhang S, Montminy M, Tan EM, Hemmerich P . (2000). CREB-binding protein (CBP)/p300 and RNA polymerase II colocalize in transcriptionally active domains in the nucleus. J Cell Biol 150: 265–273.
Wang J, Shiels C, Sasieni P, Wu PJ, Islam SA, Freemont PS et al. (2004). Promyelocytic leukemia nuclear bodies associate with transcriptionally active genomic regions. J Cell Biol 164: 515–526.
Wang Q, Ji Y, Wang X, Evers BM . (2000). Isolation and molecular characterization of the 5′-upstream region of the human TRAIL gene. Biochem Biophys Res Commun 276: 466–471.
Wang ZG, Ruggero D, Ronchetti S, Zhong S, Gaboli M, Rivi R et al. (1998). PML is essential for multiple apoptotic pathways. Nat Genet 20: 266–272.
Wu CC, Hsu SC, Shih HM, Lai MZ . (2003a). NFATc is a target of p38 mitogen activated protein kinase in T cells. Mol Cell Biol 23: 6442–6454.
Wu WS, Vallian S, Seto E, Yang WM, Edmondson D, Roth S et al. (2001). The growth suppressor PML represses transcription by functionally and physically interacting with histone deacetylases. Mol Cell Biol 21: 2259–2268.
Wu WS, Xu ZX, Hittelman WN, Salomoni P, Pandolfi PP, Chang KS . (2003b). Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-κB survival pathway. J Biol Chem 278: 12294–12304.
Wu WS, Xu ZX, Ran R, Meng F, Chang KS . (2002). Promyelocytic leukemia protein PML inhibits Nur77-mediated transcription through specific functional interactions. Oncogene 21: 3925–3933.
Youn HD, Chatila TA, Liu JO . (2000). Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J 19: 4323–4331.
Zhong S, Salomoni P, Pandolfi PP . (2000). The transcriptional role of PML and the nuclear body. Nat Cell Biol 2: E85–E90.
Zhu Y, Saunders MA, Yeh H, Deng WG, Wu KK . (2002). Dynamic regulation of cyclooxygenase-2 promoter activity by isoforms of CCAAT/enhancer-binding proteins. J Biol Chem 27: 6923–6928.
Acknowledgements
This work was supported by Grants NSC93-2320-B001-029 and NSC 94-2320-B001-012 from the National Science Council, and Grant AS-95-TP-B02-2 from Academia Sinica, Taiwan, R.O.C. We thank Dr Gerd G. Maul for PML-null MEFs, Drs Gerald Crabtree, Ron Evans, Laurie H Glimcher, Hsiou-Chi Liou and Anjana Rao for plasmids, and Dr Harry Wilson for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Lo, YH., Wu, CC., Shih, HM. et al. Selective activation of NFAT by promyelocytic leukemia protein. Oncogene 27, 3821–3830 (2008). https://doi.org/10.1038/onc.2008.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.2