Abstract
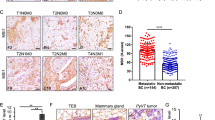
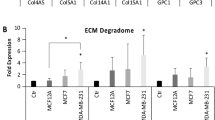
Membrane-type I matrix metalloproteinase (MT1-MMP) is associated with multiple forms of cancer including mammary cancer. To directly evaluate the significance of MT1-MMP expression in tumor progression and metastasis using a genetically induced cancer model, we crossed MT1-MMP-deficient mice to MMTV–polyoma virus middle T-antigen (PyMT) mice. Expression of PyMT in the MT1-MMP-deficient background consistently resulted in hyperplasia of the mammary gland as seen in wild-type PyMT littermates. Following orthotopic transplantation of PyMT+ glands into the cleared mammary fat pad of syngeneic recipient mice, MT1-MMP-deficient tumors were palpable earlier than wild-type tumors. Moreover, MT1-MMP-deficient tumors grew to the experimental end point size quicker than control tumors, but demonstrated markedly reduced ability to metastasize to the lungs of recipient mice. Accordingly, MT1-MMP-deficient mice displayed an overall reduction in metastasis count of 50%. MT1-MMP was expressed solely in the stroma of PyMT-induced tumors and those metastatic nodules that formed in the lungs were devoid of MT1-MMP expression. Stromal fibroblasts isolated from MT1-MMP-deficient tumors did not degrade type I collagen suggesting that efficient dissemination of tumor cells is dependent on stromal cell remodeling of the tumor environment. The data demonstrate directly that MT1-MMP-mediated proteolysis by stromal cells is important in the metastatic process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Andarawewa KL, Boulay A, Masson R, Mathelin C, Stoll I, Tomasetto C et al. (2003). Dual stromelysin-3 function during natural mouse mammary tumor virus-ras tumor progression. Cancer Res 63: 5844–5849.
Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A et al. (2003). Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet 35: 252–257.
Bisson C, Blacher S, Polette M, Blanc JF, Kebers F, Desreux J et al. (2003). Restricted expression of membrane type 1-matrix metalloproteinase by myofibroblasts adjacent to human breast cancer cells. Int J Cancer 105: 7–13.
Blavier L, Lazaryev A, Groffen J, Heisterkamp N, DeClerck YA, Kaartinen V . (2001). TGF-beta3-induced palatogenesis requires matrix metalloproteinases. Mol Biol Cell 12: 1457–1466.
Brinckerhoff CE, Matrisian LM . (2002). Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3: 207–214.
Bugge TH, Lund LR, Kombrinck KK, Nielsen BS, Holmback K, Drew AF et al. (1998). Reduced metastasis of Polyoma virus middle T antigen-induced mammary cancer in plasminogen-deficient mice. Oncogene 16: 3097–3104.
Coussens LM, Tinkle CL, Hanahan D, Werb Z . (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103: 481–490.
Dano K, Romer J, Nielsen BS, Bjorn S, Pyke C, Rygaard J et al. (1999). Cancer invasion and tissue remodeling—cooperation of protease systems and cell types. APMIS 107: 120–127.
Deryugina EI, Quigley JP . (2006). Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25: 9–34.
Egeblad M, Werb Z . (2002). New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161–174.
Gupta GP, Massague J . (2006). Cancer metastasis: building a framework. Cell 127: 679–695.
Guy CT, Cardiff RD, Muller WJ . (1992). Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12: 954–961.
Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA et al. (1999). MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: 81–92.
Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H . (2003). MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol 163: 661–671.
Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ . (2006). A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev 20: 2673–2686.
Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ . (2003). Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell 114: 33–45.
Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S . (1998). Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 58: 1048–1051.
Kalluri R, Zeisberg M . (2006). Fibroblasts in cancer. Nat Rev Cancer 6: 392–401.
Masson R, Lefebvre O, Noel A, Fahime ME, Chenard MP, Wendling C et al. (1998). In vivo evidence that the stromelysin-3 metalloproteinase contributes in a paracrine manner to epithelial cell malignancy. J Cell Biol 140: 1535–1541.
McCawley LJ, Crawford HC, King Jr LE, Mudgett J, Matrisian LM . (2004). A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res 64: 6965–6972.
Murphy G, Knauper V, Atkinson S, Butler G, English W, Hutton M et al. (2002). Matrix metalloproteinases in arthritic disease. Arthritis Res 4: S39–S49.
Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P et al. (1995). Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci USA 92: 2730–2734.
Pedersen TX, Pennington CJ, Almholt K, Christensen IJ, Nielsen BS, Edwards DR et al. (2005). Extracellular protease mRNAs are predominantly expressed in the stromal areas of microdissected mouse breast carcinomas. Carcinogenesis 26: 1233–1240.
Pendas AM, Folgueras AR, Llano E, Caterina J, Frerard F, Rodriguez F et al. (2004). Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol 24: 5304–5313.
Polette M, Gilles C, Marchand V, Seiki M, Tournier JM, Birembaut P . (1997). Induction of membrane-type matrix metalloproteinase 1 (MT1-MMP) expression in human fibroblasts by breast adenocarcinoma cells. Clin Exp Metastasis 15: 157–163.
Polette M, Nawrocki B, Gilles C, Sato H, Seiki M, Tournier JM et al. (1996). MT-MMP expression and localisation in human lung and breast cancers. Virchows Arch 428: 29–35.
Ricard-Blum S, Ruggiero F . (2005). The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 53: 430–442.
Rosenthal EL, McCrory A, Talbert M, Carroll W, Magnuson JS, Peters GE . (2004). Expression of proteolytic enzymes in head and neck cancer-associated fibroblasts. Arch Otolaryngol Head Neck Surg 130: 943–947.
Seiki M, Yana I . (2003). Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci 94: 569–574.
Stetler-Stevenson WG, Yu AE . (2001). Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol 11: 143–152.
Szabova L, Yamada SS, Birkedal-Hansen H, Holmbeck K . (2005). Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J Cell Physiol 205: 123–132.
Tran KT, Lamb P, Deng JS . (2005). Matrikines and matricryptins: implications for cutaneous cancers and skin repair. J Dermatol Sci 40: 11–20.
Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H et al. (1997). Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res 57: 2055–2060.
Vizoso FJ, Gonzalez LO, Corte MD, Rodriguez JC, Vazquez J, Lamelas ML et al. (2007). Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer 96: 903–911.
Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM . (1997). Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 94: 1402–1407.
Winkler MK, Fowlkes JL . (2002). Metalloproteinase and growth factor interactions: do they play a role in pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol 283: L1–L11.
Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI et al. (2003). Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160: 267–277.
Zatterstrom UK, Felbor U, Fukai N, Olsen BR . (2000). Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct 25: 97–101.
Acknowledgements
We thank Pamela Robey, Silvio Gutkind and Thomas Bugge for critical reading of the manuscript. This work was supported by the DIR, NIDCR of the IRP, NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Szabova, L., Chrysovergis, K., Yamada, S. et al. MT1-MMP is required for efficient tumor dissemination in experimental metastatic disease. Oncogene 27, 3274–3281 (2008). https://doi.org/10.1038/sj.onc.1210982
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210982
Keywords
This article is cited by
-
Phenotypically sorted highly and weakly migratory triple negative breast cancer cells exhibit migratory and metastatic commensalism
Breast Cancer Research (2023)
-
The role of MMP-14 in ovarian cancer: a systematic review
Journal of Ovarian Research (2021)
-
Metastasis-suppressor NME1 controls the invasive switch of breast cancer by regulating MT1-MMP surface clearance
Oncogene (2021)
-
TC10 regulates breast cancer invasion and metastasis by controlling membrane type-1 matrix metalloproteinase at invadopodia
Communications Biology (2021)
-
Less is more: low expression of MT1-MMP is optimal to promote migration and tumourigenesis of breast cancer cells
Molecular Cancer (2016)