Abstract
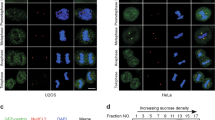
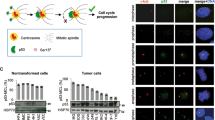
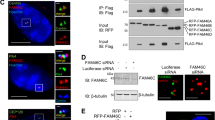
The inhibitor of DNA-binding (ID) proteins are dominant-negative inhibitors of basic helix–loop–helix transcription factors that have multiple functions during development and cellular differentiation. High-level expression of some ID family members has been observed in human malignancies, and in some cases was correlated with poor clinical prognosis. Ectopic ID1 expression extends the life span of primary human epithelial cells, inhibits cellular differentiation and induces centrosome duplication errors, thus suggesting that ID1 may have oncogenic activities. ID1 can bind to the proteasomal subunit S5A/Rpn10, but the biological consequences of the interaction have not been studied in detail. Here, we show that ID1's ability to induce supernumerary centrosomes correlates with S5A binding. Similar to ID1, a fraction of the S5A protein localizes to centrosomal structures. Furthermore, partial depletion of S5A by RNA interference causes accumulation of cells with supernumerary centrosomes. These results are consistent with the model that ID1 dysregulates centrosome homeostasis at least in part by interfering with S5A activities at the centrosome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alani RM, Hasskarl J, Grace M, Hernandez MC, Israel MA, Munger K . (1999). Immortalization of primary human keratinocytes by the helix–loop–helix protein, Id-1. Proc Natl Acad Sci USA 96: 9637–9641.
Anand G, Yin X, Shahidi AK, Grove L, Prochownik EV . (1997). Novel regulation of the helix–loop–helix protein Id1 by S5a, a subunit of the 26 S proteasome. J Biol Chem 272: 19140–19151.
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M . (2003). Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426: 570–574.
Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H . (1990). The protein Id: a negative regulator of helix–loop–helix DNA binding proteins. Cell 61: 49–59.
Berse M, Bounpheng M, Huang X, Christy B, Pollmann C, Dubiel W . (2004). Ubiquitin-dependent degradation of Id1 and Id3 is mediated by the COP9 signalosome. J Mol Biol 343: 361–370.
Bloom J, Peschiaroli A, Demartino G, Pagano M . (2006). Modification of Cul1 regulates its association with proteasomal subunits. Cell Div 1: 5.
Bounpheng MA, Dimas JJ, Dodds SG, Christy BA . (1999). Degradation of Id proteins by the ubiquitin–proteasome pathway. FASEB J 13: 2257–2264.
Brummelkamp TR, Bernards R, Agami R . (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553.
D’Assoro AB, Stivala F, Barrett S, Ferrigno G, Salisbury JL . (2001). GFP-centrin as a marker for centriole dynamics in the human breast cancer cell line MCF-7. Ital J Anat Embryol 106: 103–110.
Deed RW, Hara E, Atherton GT, Peters G, Norton JD . (1997). Regulation of Id3 cell cycle function by Cdk-2-dependent phosphorylation. Mol Cell Biol 17: 6815–6821.
Doxsey SJ . (2001). Centrosomes as command centres for cellular control. Nat Cell Biol 3: E105–E108.
Duensing S, Duensing A, Crum CP, Munger K . (2001). Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res 61: 2356–2360.
Duensing S, Duensing A, Lee DC, Edwards KM, Piboonniyom SO, Manuel E et al (2004). Cyclin-dependent kinase inhibitor indirubin-3′-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies. Oncogene 23: 8206–8215.
Duensing S, Lee BH, Cin PD, Munger K . (2003). Excessive centrosome abnormalities without ongoing numerical chromosome instability in a Burkitt's lymphoma. Mol Cancer 2: 30.
Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S et al (2000). The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA 97: 10002–10007.
Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN . (2000). Activity and regulation of the centrosome-associated proteasome. J Biol Chem 275: 409–413.
Fajerman I, Schwartz AL, Ciechanover A . (2004). Degradation of the Id2 developmental regulator: targeting via N-terminal ubiquitination. Biochem Biophys Res Commun 314: 505–512.
Florio M, Hernandez MC, Yang H, Shu HK, Cleveland JL, Israel MA . (1998). Id2 promotes apoptosis by a novel mechanism independent of dimerization to basic helix–loop–helix factors. Mol Cell Biol 18: 5435–5444.
Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT et al (2004). Rescue of cardiac defects in ID knockout embryos by injection of embryonic stem cells. Science 306: 247–252.
Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T et al (1999). Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev 13: 2242–2257.
Goldberg AL . (2003). Protein degradation and protection against misfolded or damaged proteins. Nature 426: 895–899.
Hara E, Hall M, Peters G . (1997). Cdk2-dependent phosphorylation of ID2 modulates activity of E2A-related transcription factors. EMBO J 16: 332–342.
Hara E, Uzman JA, Dimri GP, Nehlin JO, Testori A, Campisi J . (1996). The helix–loop–helix protein Id-1 and a retinoblastoma protein binding mutant of SV40T antigen synergize to reactivate DNA synthesis in senescent human fibroblasts. Dev Genet 18: 161–172.
Hasskarl J, Duensing S, Manuel E, Munger K . (2004). The helix–loop–helix protein ID1 localizes to centrosomes and rapidly induces abnormal centrosome numbers. Oncogene 63: 476–483.
Hinchcliffe EH, Sluder G . (2001). ‘It takes two to tango’: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev 15: 1167–1181.
Hofmann K, Falquet L . (2001). A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci 26: 347–350.
Iavarone A, Garg P, Lasorella A, Hsu J, Israel MA . (1994). The helix–loop–helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev 8: 1270–1284.
Jen Y, Weintraub H, Benezra R . (1992). Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev 6: 1466–1479.
Kreider BL, Benezra R, Rovera G, Kadesch T . (1992). Inhibition of myeloid differentiation by the helix–loop–helix protein Id. Science 255: 1700–1702.
Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G et al (2006). Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 442: 471–474.
Ling MT, Wang X, Ouyang XS, Xu K, Tsao SW, Wong YC . (2003). Id-1 expression promotes cell survival through activation of NF-kappaB signalling pathway in prostate cancer cells. Oncogene 22: 4498–4508.
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L et al (2001). Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7: 1194–1201.
Meraldi P, Nigg EA . (2002). The centrosome cycle. FEBS Lett 521: 9–13.
Nickoloff BJ, Chaturvedi V, Bacon P, Qin JZ, Denning MF, Diaz MO . (2000). Id-1 delays senescence but does not immortalize keratinocytes. J Biol Chem 275: 27501–27504.
Ohtani N, Zebedee Z, Huot TJ, Stinson JA, Sugimoto M, Ohashi Y et al (2001). Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409: 1067–1070.
Perk J, Iavarone A, Benezra R . (2005). Id family of helix–loop–helix proteins in cancer. Nat Rev Cancer 5: 603–614.
Roberts EC, Deed RW, Inoue T, Norton JD, Sharrocks AD . (2001). Id helix–loop–helix proteins antagonize pax transcription factor activity by inhibiting DNA binding. Mol Cell Biol 21: 524–533.
Stavropoulou V, Xie J, Henriksson M, Tomkinson B, Imreh S, Masucci MG . (2005). Mitotic infidelity and centrosome duplication errors in cells overexpressing tripeptidyl-peptidase II. Cancer Res 65: 1361–1368.
Storchova Z, Pellman D . (2004). From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol 5: 45–54.
Szlanka T, Haracska L, Kiss I, Deak P, Kurucz E, Ando I et al (2003). Deletion of proteasomal subunit S5a/Rpn10/p54 causes lethality, multiple mitotic defects and overexpression of proteasomal genes in Drosophila melanogaster. J Cell Sci 116: 1023–1033.
Tang J, Gordon GM, Nickoloff BJ, Foreman KE . (2002). The helix–loop–helix protein id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest 82: 1073–1079.
Trausch-Azar JS, Lingbeck J, Ciechanover A, Schwartz AL . (2004). Ubiquitin–proteasome-mediated degradation of Id1 is modulated by MyoD. J Biol Chem 279: 32614–32619.
Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN et al (1999). Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 145: 481–490.
Ying QL, Nichols J, Chambers I, Smith A . (2003). BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115: 281–292.
Acknowledgements
We thank M Bornens for the centrin-GFP construct, R Agami for pSUPER.retro, PM Howley for S5A cDNA, and S Duensing for helpful discussion. We thank N Bourgeois for technical assistance. JH is especially grateful for the extraordinary support of K Hasskarl. This work was supported by Public Health Service Grant R01 CA066980 (KM), by postdoctoral fellowship HA3185/1-1 and research Grant HA3185/2-1 and 2-3 from the Deutsche Forschungsgemeinschaft (JH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Hasskarl, J., Mern, D. & Münger, K. Interference of the dominant negative helix–loop–helix protein ID1 with the proteasomal subunit S5A causes centrosomal abnormalities. Oncogene 27, 1657–1664 (2008). https://doi.org/10.1038/sj.onc.1210808
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210808
Keywords
This article is cited by
-
The Id-protein family in developmental and cancer-associated pathways
Cell Communication and Signaling (2017)
-
A reduced VWA domain-containing proteasomal ubiquitin receptor of Giardia lamblia localizes to the flagellar pore regions in microtubule-dependent manner
Parasites & Vectors (2015)
-
Elevated endogenous expression of the dominant negative basic helix-loop-helix protein ID1 correlates with significant centrosome abnormalities in human tumor cells
BMC Cell Biology (2010)
-
A novel role of the aryl hydrocarbon receptor (AhR) in centrosome amplification - implications for chemoprevention
Molecular Cancer (2010)