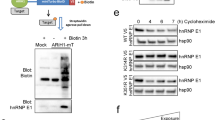
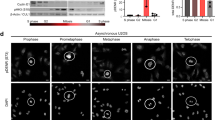
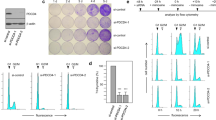
Abstract
Regulation of the synthesis, function and degradation of HDM2 (Mdm2 in mouse) plays a key role in controlling the abundance and activity of the transcription factor p53, with consequent implications for the proliferation and survival of normal and cancer cells. We have previously identified the regulation of export of HDM2 mRNA from the nucleus as a novel point of control of HDM2 synthesis. This process is dependent on the activity of the growth factor-regulated MAP-kinase kinases (MEKs). Here, we provide evidence that the eIF4E kinase MNK1 is a key downstream effector of MEKs in this regulatory pathway. We show that HDM2 mRNA export in breast cancer cells is promoted by overexpressed eIF4E in a MEK- and MNK1-dependent manner, and inhibition of MNK1 suppresses endogenous HDM2 mRNA export pathways. This MNK1- and eIF4E-dependent HDM2 regulation occurs through sequences in the 3′ untranslated region of HDM2 mRNA, and consequently HDM2 mRNA transcripts from both the constitutive P1 and inducible P2 promoters are regulated by this pathway. eIF4E is a known oncogene that is overexpressed in human tumours, including the majority of breast cancers. This pathway, therefore, may play an important role in the dysregulation of HDM2 oncoprotein expression that occurs in many human tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blaydes JP, Gire V, Rowson J, Wynford-Thomas D . (1997). Tolerance of high levels of wild-type p53 in transformed epithelial cells dependent on auto-regulation by mdm-2. Oncogene 14: 1859–1868.
Blaydes JP, Wynford-Thomas D . (1998). The proliferation of normal human fibroblasts is dependent upon negative regulation of p53 function by mdm2. Oncogene 16: 3317–3322.
Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC et al. (2004). A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119: 591–602.
Bond GL, Levine AJ . (2007). A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene 26: 1317–1323.
Brooks CL, Gu W . (2006). p53 ubiquitination: Mdm2 and beyond. Mol Cell 21: 307–315.
Buxade M, Parra JL, Rousseau S, Shpiro N, Marquez R, Morrice N et al. (2005). The Mnks are novel components in the control of TNF alpha biosynthesis and phosphorylate and regulate hnRNP A1. Immunity 23: 177–189.
Chen J, Marechal V, Levine AJ . (1993). Mapping of the p53 and mdm-2 interaction domains. Mol Cell Biol 13: 4107–4114.
Culjkovic B, Topisirovic I, Borden KL . (2007). Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell Cycle 6: 65–69.
Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL . (2005). eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol 169: 245–256.
Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL . (2006). eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol 175: 415–426.
Cullen BR . (2003). Nuclear RNA export. J Cell Sci 116: 587–597.
Erkmann JA, Kutay U . (2004). Nuclear export of mRNA: from the site of transcription to the cytoplasm. Exp Cell Res 296: 12–20.
Gallouzi IE, Steitz JA . (2001). Delineation of mRNA export pathways by the use of cell-permeable peptides. Science 294: 1895–1901.
Harvey M, Sands AT, Weiss RS, Hegi ME, Wiseman RW, Pantazis P et al. (1993). In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 8: 2457–2467.
Keene JD . (2003). Organizing mRNA export. Nat Genet 33: 111–112.
Knauf U, Tschopp C, Gram H . (2001). Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol 21: 5500–5511.
Mamane Y, Petroulakis E, Martineau Y, Sato TA, Larsson O, Rajasekhar VK et al. (2007). Epigenetic activation of a subset of mRNAs by eIF4E explains its effects on cell proliferation. PLoS ONE 2: e242.
Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N . (2004). eIF4E—from translation to transformation. Oncogene 23: 3172–3179.
Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B . (1992). Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature (London) 358: 80–83.
Onel K, Cordon-Cardo C . (2004). MDM2 and prognosis. Mol Cancer Res 2: 1–8.
Phelps M, Darley M, Primrose JN, Blaydes JP . (2003). p53-independent activation of the hdm2-P2 promoter through multiple transcription factor response elements results in elevated hdm2 expression in estrogen receptor alpha positive breast cancer cells. Cancer Res 63: 2616–2623.
Phelps M, Phillips A, Darley M, Blaydes JP . (2005). MEK-ERK signaling controls Hdm2 oncoprotein expression by regulating hdm2 mRNA export to the cytoplasm. J Biol Chem 280: 16651–16658.
Phillips A, Darley M, Blaydes JP . (2006a). GC-selective DNA-binding antibiotic, Mithramycin A, reveals multiple points of control in the regulation of Hdm2 protein synthesis. Oncogene 25: 4183–4193.
Phillips A, Jones CJ, Blaydes JP . (2006b). The mechanisms of regulation of Hdm2 protein level by serum growth factors. FEBS Lett 580: 300–304.
Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T et al. (2000). Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell 103: 321–330.
Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N . (1996). Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA 93: 1065–1070.
Shaulian E, Resnitzky D, Shifman O, Blandino G, Amsterdam A, Yayon A et al. (1997). Induction of Mdm2 and enhancement of cell survival by bFGF. Oncogene 15: 2717–2725.
Topisirovic I, Guzman ML, McConnell MJ, Licht JD, Culjkovic B, Neering SJ et al. (2003). Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol 23: 8992–9002.
Topisirovic I, Ruiz-Gutierrez M, Borden KL . (2004). Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res 64: 8639–8642.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. (2004). In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848.
Vogelstein B, Lane D, Levine AJ . (2000). Surfing the p53 network. Nature 408: 307–310.
von der Haar T, Gross JD, Wagner G, McCarthy JE . (2004). The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol 11: 503–511.
Wahl GM, Carr AM . (2001). The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol 3: E277–E286.
Wallace M, Worrall E, Pettersson S, Hupp TR, Ball KL . (2006). Dual-site regulation of MDM2 E3-ubiquitin ligase activity. Mol Cell 23: 251–263.
Waskiewicz AJ, Flynn A, Proud CG, Cooper JA . (1997). Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J 16: 1909–1920.
Zauberman A, Flusberg D, Barak Y, Oren M . (1995). A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic Acids Res 23: 2584–2592.
Acknowledgements
This work was supported by a grant (no. 04-422) from the Association for International Cancer Research. We are grateful to Professor Katherine Borden for the sharing of data prior to its publication, N Sonenberg for making available the eIF4E expression vector and Dr Monika Phelps for cloning of the HDM2 3′UTR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phillips, A., Blaydes, J. MNK1 and EIF4E are downstream effectors of MEKs in the regulation of the nuclear export of HDM2 mRNA. Oncogene 27, 1645–1649 (2008). https://doi.org/10.1038/sj.onc.1210785
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210785
Keywords
This article is cited by
-
Characterization of MNK1b DNA Aptamers That Inhibit Proliferation in MDA-MB231 Breast Cancer Cells
Molecular Therapy - Nucleic Acids (2016)
-
Oncogenic Ras inhibits IRF1 to promote viral oncolysis
Oncogene (2015)
-
Oncogenic Activation of MEK/ERK Primes Melanoma Cells for Adaptation to Endoplasmic Reticulum Stress
Journal of Investigative Dermatology (2014)
-
Repression of microRNA-768-3p by MEK/ERK signalling contributes to enhanced mRNA translation in human melanoma
Oncogene (2014)