Abstract
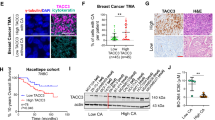
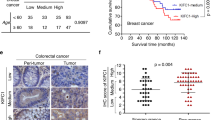
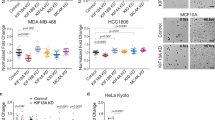
Regulators of the mitotic spindle apparatus are attractive cellular targets for antitumor therapy. The centrosomal protein transforming acidic coiled coil (TACC) 3 is required for spindle assembly and proper chromosome segregation. In this study, we employed an inducible RNA interference approach to downregulate TACC3 expression. We show that TACC3 knock-down in NIH3T3 fibroblasts caused aneuploidy, but failed to overtly impair mitotic progression. TACC3 depletion rather triggered a postmitotic p53–p21WAF pathway and led to a reversible cell cycle arrest. Similar effects were induced by low concentrations of paclitaxel, a spindle poison used in antitumor therapy. Interestingly, however, and unlike in TACC3-proficient cells, paclitaxel was able to induce strong polyploidy and subsequent apoptosis in TACC3-depleted cells. Even though paclitaxel treatment was associated with the activation of the survival kinase Akt and an antiapoptotic expression of cytoplasmic p21WAF and cyclin D1, this inhibition of cell death was abrogated by depletion of TACC3. Thus, our data identify TACC3 as a potential target to overcome p21WAF-associated protection of transformed cells against paclitaxel-induced cell death.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abal M, Andreu JM, Barasoain I . (2003). Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets 3: 193–203.
Andreassen PR, Lohez OD, Margolis RL . (2003). G2 and spindle assembly checkpoint adaptation, and tetraploidy arrest: implications for intrinsic and chemically induced genomic instability. Mutat Res 532: 245–253.
Blagosklonny MV . (2002). Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle 1: 391–393.
Blagosklonny MV . (2006). Prolonged mitosis versus tetraploid checkpoint: how p53 measures the duration of mitosis. Cell Cycle 5: 971–975.
Blagosklonny MV, Darzynkiewicz Z, Halicka HD, Pozarowski P, Demidenko ZN, Barry JJ et al. (2004). Paclitaxel induces primary and postmitotic G1 arrest in human arterial smooth muscle cells. Cell Cycle 3: 1050–1056.
Blagosklonny MV, Demidenko ZN, Giovino M, Szynal C, Donskoy E, Herrmann RA et al. (2006). Cytostatic activity of paclitaxel in coronary artery smooth muscle cells is mediated through transient mitotic arrest followed by permanent post-mitotic arrest: comparison with cancer cells. Cell Cycle 5: 1574–1579.
Blagosklonny MV, Robey R, Bates S, Fojo T . (2000). Pretreatment with DNA-damaging agents permits selective killing of checkpoint-deficient cells by microtubule-active drugs. J Clin Invest 105: 533–539.
Brito DA, Rieder CL . (2006). Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol 16: 1194–1200.
Cassimeris L, Morabito J . (2004). TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol Biol Cell 15: 1580–1590.
Chen JG, Horwitz SB . (2002). Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res 62: 1935–1938.
Chen X, Bargonetti J, Prives C . (1995). p53, through p21 (WAF1/CIP1), induces cyclin D1 synthesis. Cancer Res 55: 4257–4263.
Child ES, Mann DJ . (2006). The intricacies of p21 phosphorylation. Cell Cycle 5: 1313–1319.
Coqueret O . (2003). New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13: 65–70.
Cully M, Shiu J, Piekorz RP, Muller WJ, Done SJ, Mak TW . (2005). Transforming acidic coiled coil 1 promotes transformation and mammary tumorigenesis. Cancer Res 65: 10363–10370.
Del Sal G, Murphy M, Ruaro E, Lazarevic D, Levine AJ, Schneider C . (1996). Cyclin D1 and p21/waf1 are both involved in p53 growth suppression. Oncogene 12: 177–185.
Gergely F . (2002). Centrosomal TACCtics. BioEssays 24: 915–925.
Gergely F, Draviam VM, Raff JW . (2003). The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev 17: 336–341.
Giannakakou P, Robey R, Fojo T, Blagosklonny MV . (2001). Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 20: 3806–3813.
Gladden AB, Diehl JA . (2005). Location, location, location: the role of cyclin D1 nuclear localization in cancer. J Cell Biochem 96: 906–913.
Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD . (2000). CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103: 435–447.
Han EK, Ng SC, Arber N, Begemann M, Weinstein IB . (1999). Roles of cyclin D1 and related genes in growth inhibition, senescence and apoptosis. Apoptosis 4: 213–219.
Heliez C, Baricault L, Barboule N, Valette A . (2003). Paclitaxel increases p21 synthesis and accumulation of its AKT-phosphorylated form in the cytoplasm of cancer cells. Oncogene 22: 3260–3268.
Ikui AE, Yang CP, Matsumoto T, Horwitz SB . (2005). Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle 4: 1385–1388.
Janicke RU, Sohn D, Essmann F, Schulze-Osthoff K . (2007). The multiple battles fought by anti-apoptotic p21. Cell Cycle 6: 407–413.
Jordan MA, Wilson L . (2004). Microtubules as a target for anticancer drugs. Nat Rev Cancer 4: 253–265.
Judson PL, Watson JM, Gehrig PA, Fowler Jr WC, Haskill JS . (1999). Cisplatin inhibits paclitaxel-induced apoptosis in cisplatin-resistant ovarian cancer cell lines: possible explanation for failure of combination therapy. Cancer Res 59: 2425–2432.
Jung CK, Jung JH, Park GS, Lee A, Kang CS, Lee KY . (2006). Expression of transforming acidic coiled-coil containing protein 3 is a novel independent prognostic marker in non-small cell lung cancer. Pathol Int 56: 503–509.
Kadura S, Sazer S . (2005). SAC-ing mitotic errors: how the spindle assembly checkpoint (SAC) plays defense against chromosome mis-segregation. Cell Motil Cytoskeleton 61: 145–1460.
Lauffart B, Vaughan MM, Eddy R, Chervinsky D, DiCioccio RA, Black JD et al. (2005). Aberrations of TACC1 and TACC3 are associated with ovarian cancer. BMC Women's Health 5: 8.
Leurs C, Jansen M, Pollok KE, Heinkelein M, Schmidt M, Wissler M et al. (2003). Comparison of three retroviral vector systems for transduction of nonobese diabetic/severe combined immunodeficiency mice repopulating human CD34+ cord blood cells. Hum Gene Ther 14: 509–519.
Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S . (2007). Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol 9: 160–170.
Nurse P . (1990). Universal control mechanism regulating onset of M-phase. Nature 344: 503–508.
Olashaw N, Bagui TK, Pledger WJ . (2004). Cell cycle control: a complex issue. Cell Cycle 3: 263–264.
Piekorz RP, Hoffmeyer A, Duntsch CD, McKay C, Nakajima H, Sexl V et al. (2002). The centrosomal protein TACC3 is essential for hematopoietic stem cell function and genetically interfaces with p53-regulated apoptosis. EMBO J 21: 653–664.
Schmidt M, Lu Y, Liu B, Fang M, Mendelsohn J, Fan Z . (2000). Differential modulation of paclitaxel-mediated apoptosis by p21Waf1 and p27Kip1. Oncogene 19: 2423–2429.
Sohn D, Essmann F, Schulze-Osthoff K, Janicke RU . (2006). p21 blocks irradiation-induced apoptosis downstream of mitochondria by inhibition of cyclin-dependent kinase-mediated caspase-9 activation. Cancer Res 66: 11254–11262.
Srsen V, Gnadt N, Dammermann A, Merdes A . (2006). Inhibition of centrosome protein assembly leads to p53-dependent exit from the cell cycle. J Cell Biol 174: 625–630.
Stewart ZA, Mays D, Pietenpol JA . (1999). Defective G1-S cell cycle checkpoint function sensitizes cells to microtubule inhibitor-induced apoptosis. Cancer Res 59: 3831–3837.
Still IH, Hamilton M, Vince P, Wolfman A, Cowell JK . (1999a). Cloning of TACC1, an embryonically expressed, potentially transforming coiled coil containing gene, from the 8p11 breast cancer amplicon. Oncogene 18: 4032–4038.
Still IH, Vince P, Cowell JK . (1999b). The third member of the transforming acidic coiled coil-containing gene family, TACC3, maps in 4p16, close to translocation breakpoints in multiple myeloma, and is upregulated in various cancer cell lines. Genomics 58: 165–170.
Taylor WR, Stark GR . (2001). Regulation of the G2/M transition by p53. Oncogene 20: 1803–1815.
Vogel C, Kienitz A, Hofmann I, Muller R, Bastians H . (2004). Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene 23: 6845–6853.
West KA, Castillo SS, Dennis PA . (2002). Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 5: 234–248.
Wiznerowicz M, Trono D . (2003). Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol 77: 8957–8961.
Yao R, Natsume Y, Noda T . (2007). TACC3 is required for the proper mitosis of sclerotome mesenchymal cells during formation of the axial skeleton. Cancer Sci 98: 555–562.
Yu J, Zhang L . (2005). The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331: 851–858.
Zachos G, Black EJ, Walker M, Scott M, Vagnarelli P, Earnshaw WC et al. (2007). Chk1 is required for spindle checkpoint function. Dev Cell 12: 247–260.
Acknowledgements
We thank Didier Trono (Swiss Federal Institute of Technology, Laussane) and Lars-Oliver Klotz (Institut für Umweltmedizinische Forschung, Düsseldorf) for kindly providing constructs for lentivector-mediated RNA interference and antibodies, respectively. We are indebted to Antje Gohla, Reiner U Jänicke, Mohamad Ahmadian, Chris Wichmann and members of the department for critical comments on the manuscript. This work was supported by intramural grant 9772210 from the Medical Faculty of the Heinrich-Heine-University Düsseldorf (to RPP) and by the Deutsche Forschungsgemeinschaft (to HH, KSO, BN and RPP), by the Deutsche Jose Carreras Stiftung (to HH) and by Fonds der Chemischen Industrie (to KSO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Schneider, L., Essmann, F., Kletke, A. et al. TACC3 depletion sensitizes to paclitaxel-induced cell death and overrides p21WAF-mediated cell cycle arrest. Oncogene 27, 116–125 (2008). https://doi.org/10.1038/sj.onc.1210628
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210628
Keywords
This article is cited by
-
Functional proteomic analysis reveals the involvement of KIAA1199 in breast cancer growth, motility and invasiveness
BMC Cancer (2014)
-
Cancer cell death induced by novel small molecules degrading the TACC3 protein via the ubiquitin–proteasome pathway
Cell Death & Disease (2014)
-
Disruption of Tacc3 function leads to in vivo tumor regression
Oncogene (2012)
-
The centrosomal protein TACC3 controls paclitaxel sensitivity by modulating a premature senescence program
Oncogene (2010)