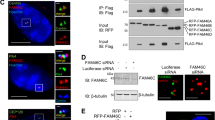
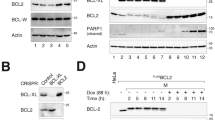
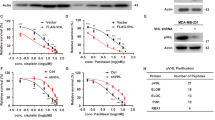
Abstract
E2F-1 controls multiple cellular activities through transcriptional regulation of its target genes. As a mediator of cell death, E2F-1 can eliminate latent neoplastic cells through apoptosis. However, the mechanism by which E2F-1 mediates cancer cell killing is largely unknown. In this paper, we report that phosphatase of activated cells 1 (PAC1) phosphatase is a direct transcription target of E2F-1 in signaling apoptosis. We show that ectopic E2F-1 increases expression of PAC1 at both transcriptional and translational levels in breast cancer cells. E2F-1 physically interacts with the promoter of PAC1, binds to its consensus sequence in the promoter and transactivates the PAC1 promoter. E2F-1 suppresses extracellular signal-regulated kinase (ERK) phosphorylation through PAC1 and causes cancer cell death by apoptosis following treatment with a chemotherapeutic agent N-4-hydroxyphenylretinamide (4-HPR). Furthermore, ectopic PAC1 inhibits ERK phosphorylation and mediates cell killing. Moreover, endogenous E2F-1 upregulates PAC1 and suppresses ERK activity, leading to cell death in response to 4-HPR. These results reveal a crucial role of PAC1 in E2F-1-directed apoptosis. Our study demonstrates that E2F-1 mediates apoptosis through transcriptional regulation of PAC1 and subsequent suppression of the ERK signaling. Our findings establish a functional link between E2F-1 and mitogen-activated protein kinases. The E2F-1–PAC1 cascade in cancer cell killing may provide a molecular basis for cancer therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bednarek A, Shilkaitis A, Green A, Lubet R, Kelloff G, Christov K et al. (1999). Suppression of cell proliferation and telomerase activity in 4-(hydroxyphenyl)retinamide-treated mammary tumors. Carcinogenesis 20: 879–883.
Bell LA, Ryan KM . (2004). Life and death decisions by E2F-1. Cell Death Differ 11: 137–142.
Cam H, Dynlacht BD . (2003). Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell 3: 311–316.
Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K . (1996). The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem 271: 6497–6501.
Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML et al. (1993). Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362: 849–852.
Conklin KA . (2004). Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther 3: 294–300.
Creagh EM, Conroy H, Martin SJ . (2003). Caspase activation pathways in apoptosis and immunity. Immunol Rev 193: 10–21.
DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR . (1997). Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci USA 94: 7245–7250.
Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin Jr WG, Livingston DM et al. (1996). E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85: 549–561.
Furukawa Y, Nishimura N, Satoh M, Endo H, Iwase S, Yamada H et al. (2002). Apaf-1 is a mediator of E2F-1-induced apoptosis. J Biol Chem 277: 39760–39768.
Ginsberg D . (2002). E2F1 pathways to apoptosis. FEBS Lett 529: 122–125.
Huang LC, Clarkin KC, Wahl GM . (1996). Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc Natl Acad Sci USA 93: 4827–4832.
Husain SS, Szabo IL, Pai R, Soreghan B, Jones MK, Tarnawski AS . (2001). MAPK (ERK2) kinase – a key target for NSAIDs-induced inhibition of gastric cancer cell proliferation and growth. Life Sci 69: 3045–3054.
Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W et al. (2000). Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 407: 645–648.
Kim HJ, Chakravarti N, Oridate N, Choe C, Claret FX, Lotan R . (2006). N-(4-hydroxyphenyl)retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene 25: 2785–2794.
Lewis TS, Shapiro PS, Ahn NG . (1998). Signal transduction through MAP kinase cascades. Adv Cancer Res 74: 49–139.
Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF . (2000). A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 407: 642–645.
Malone W, Perloff M, Crowell J, Sigman C, Higley H . (2003). Fenretinide: a prototype cancer prevention drug. Expert Opin Investig Drugs 12: 1829–1842.
Mates JM, Sanchez-Jimenez FM . (2000). Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol 32: 157–170.
Miyata Y, Nishida E . (1999). Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem Biophys Res Commun 266: 291–295.
Moon RC, Mehta RG . (1989). Chemoprevention of experimental carcinogenesis in animals. Prev Med 18: 576–591.
Ookawa K, Tsuchida S, Adachi J, Yokota J . (1997). Differentiation induced by RB expression and apoptosis induced by p53 expression in an osteosarcoma cell line. Oncogene 14: 1389–1396.
Rohan PJ, Davis P, Moskaluk CA, Kearns M, Krutzsch H, Siebenlist U et al. (1993). PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science 259: 1763–1766.
Shen WH, Wang J, Wu J, Zhurkin VB, Yin Y . (2006). Mitogen-activated protein kinase phosphatase 2: a novel transcription target of p53 in apoptosis. Cancer Res 66: 6033–6039.
Shen WH, Yin Y, Broussard SR, McCusker RH, Freund GG, Dantzer R et al. (2004). Tumor necrosis factor alpha inhibits cyclin A expression and retinoblastoma hyperphosphorylation triggered by insulin-like growth factor-I induction of new E2F-1 synthesis. J Biol Chem 279: 7438–7446.
Simeone AM, Broemeling LD, Rosenblum J, Tari AM . (2003). HER2/neu reduces the apoptotic effects of N-(4-hydroxyphenyl)retinamide (4-HPR) in breast cancer cells by decreasing nitric oxide production. Oncogene 22: 6739–6747.
Simeone AM, Ekmekcioglu S, Broemeling LD, Grimm EA, Tari AM . (2002). A novel mechanism by which N-(4-hydroxyphenyl)retinamide inhibits breast cancer cell growth: the production of nitric oxide. Mol Cancer Ther 1: 1009–1017.
Sun H, Charles CH, Lau LF, Tonks NK . (1993). MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487–493.
Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E et al. (2002). A p53–p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene 21: 3872–3878.
Ward Y, Gupta S, Jensen P, Wartmann M, Davis RJ, Kelly K . (1994). Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature 367: 651–654.
Whitmarsh AJ, Davis RJ . (1999). Signal transduction by MAP kinases: regulation by phosphorylation-dependent switches. Sci STKE 1999: PE1.
Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ . (1996). Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85: 537–548.
Yan W, Chen X . (2006). GPX2, a direct target of p63, inhibits oxidative stress-induced apoptosis in a p53-dependent manner. J Biol Chem 281: 7856–7862.
Yin Y, Liu YX, Jin YJ, Hall EJ, Barrett JC . (2003). PAC1 phosphatase is a transcription target of p53 in signalling apoptosis and growth suppression. Nature 422: 527–531.
Yin Y, Solomon G, Deng C, Barrett JC . (1999). Differential regulation of p21 by p53 and Rb in cellular response to oxidative stress. Mol Carcinog 24: 15–24.
Yin Y, Terauchi Y, Solomon GG, Aizawa S, Rangarajan PN, Yazaki Y et al. (1998). Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature 391: 707–710.
Zhang Q, Muller M, Chen CH, Zeng L, Farooq A, Zhou MM . (2005). New insights into the catalytic activation of the MAPK phosphatase PAC-1 induced by its substrate MAPK ERK2 binding. J Mol Biol 354: 777–788.
Zhu L, Enders GH, Wu CL, Starz MA, Moberg KH, Lees JA et al. (1994). Growth suppression by members of the retinoblastoma protein family. Cold Spring Harb Symp Quant Biol 59: 75–84.
Acknowledgements
We are grateful to R Baer, HB Lieberman, and PW Brandt-Rauf for critical comments on the manuscript. We thank WH Shen for manuscript preparation and data analysis. W Huang was supported by the Hi-Tech Research and Development Program of China (2006AA02Z489, 2004CB518801). This work was supported by an NIH grant (CA102447) to YY.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, J., Jin, Y., Calaf, G. et al. PAC1 is a direct transcription target of E2F-1 in apoptotic signaling. Oncogene 26, 6526–6535 (2007). https://doi.org/10.1038/sj.onc.1210484
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210484