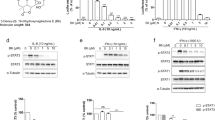
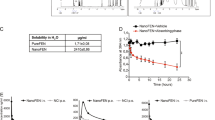
Abstract
Indirubin-3′-monoxime is a derivative of the bis-indole alkaloid indirubin, an active ingredient of a traditional Chinese medical preparation that exhibits anti-inflammatory and anti-leukemic activities. Indirubin-3′-monoxime is mainly recognized as an inhibitor of cyclin-dependent kinases (CDKs) and glycogen synthase kinase-3. It inhibits proliferation of cultured cells, mainly through arresting the cells in the G1/S or G2/M phase of the cell cycle. Here, we report that indirubin-3′-monoxime is able to inhibit proliferation of NIH/3T3 cells by specifically inhibiting autophosphorylation of fibroblast growth factor receptor 1 (FGFR1), blocking in this way the receptor-mediated cell signaling. Indirubin-3′-monoxime inhibits the activity of FGFR1 at a concentration lower than that required for inhibition of phosphorylation of CDK2 and retinoblastoma protein and cell proliferation stimulated by fetal calf serum. The ability of indirubin-3′-monoxime to inhibit FGFR1 signaling was similar to that of the FGFR1 inhibitor SU5402. In addition, we found that indirubin-3′-monoxime activates long-term p38 mitogen-activated protein kinase activity, which stimulates extracellular signal-regulated kinase 1/2 in a way unrelated to the activity of FGFR1. Furthermore, we show that indirubin-3′-monoxime can inhibit proliferation of the myeloid leukemia cell line KG-1a through inhibition of the activity of the FGFR1 tyrosine kinase. The data presented here demonstrate previously unknown activities of indirubin-3′-monoxime that may have clinical implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- AML:
-
acute myeloid leukemia
- CDK:
-
cyclin-dependent kinase
- FCS:
-
fetal calf serum
- FGFR:
-
FGF receptor
- HSPG:
-
heparan sulfate proteoglycan
- JNK:
-
c-Jun N -terminal kinase
- Rb:
-
retinoblastoma protein
- TCA:
-
trichloroacetic acid
References
Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H et al. (2001). Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem 276: 31475–31478.
Benhar M, Dalyot I, Engelberg D, Levitzki A . (2001). Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol 21: 6913–6926.
Boilly B, Vercoutter-Edouart AS, Hondermarck H, Nurcombe V, Le BX . (2000). FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev 11: 295–302.
Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y et al. (2003). Mechanism of p38 MAP kinase activation in vivo. Genes Dev 17: 1969–1978.
Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E et al. (1999). Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18: 6845–6854.
Chaffanet M, Popovici C, Leroux D, Jacrot M, Adelaide J, Dastugue N et al. (1998). t(6;8), t(8;9) and t(8;13) translocations associated with stem cell myeloproliferative disorders have close or identical breakpoints in chromosome region 8p11–12. Oncogene 16: 945–949.
Chiariello M, Gomez E, Gutkind JS . (2000). Regulation of cyclin-dependent kinase (Cdk) 2 Thr-160 phosphorylation and activity by mitogen-activated protein kinase in late G1 phase. Biochem J 349 (Part 3): 869–876.
Dailey L, Ambrosetti D, Mansukhani A, Basilico C . (2005). Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16: 233–247.
Davies TG, Tunnah P, Meijer L, Marko D, Eisenbrand G, Endicott JA et al. (2001). Inhibitor binding to active and inactive CDK2: the crystal structure of CDK2-cyclin A/indirubin-5-sulphonate. Structure 9: 389–397.
Eswarakumar VP, Lax I, Schlessinger J . (2005). Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16: 139–149.
Fraker PJ, Speck Jr JC . (1978). Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun 80: 849–857.
Furdui CM, Lew ED, Schlessinger J, Anderson KS . (2006). Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol Cell 21: 711–717.
Gu TL, Goss VL, Reeves C, Popova L, Nardone J, Macneill J et al. (2006). Phosphotyrosine profiling identifies the KG-1 cell line as a model for the study of FGFR1 fusions in acute myeloid leukemia. Blood 108: 4202–4204.
Guasch G, Ollendorff V, Borg JP, Birnbaum D, Pebusque MJ . (2001). 8p12 stem cell myeloproliferative disorder: the FOP-fibroblast growth factor receptor 1 fusion protein of the t(6;8) translocation induces cell survival mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt/mTOR pathways. Mol Cell Biol 21: 8129–8142.
Heredia A, Davis C, Bamba D, Le N, Gwarzo MY, Sadowska M et al. (2005). Indirubin-3′-monoxime, a derivative of a Chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication. AIDS 19: 2087–2095.
Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P et al. (1999). Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol 1: 60–67.
Imamura T, Oka S, Tanahashi T, Okita Y . (1994). Cell cycle-dependent nuclear localization of exogenously added fibroblast growth factor-1 in BALB/c 3T3 and human vascular endothelial cells. Exp Cell Res 215: 363–372.
Itoh N, Ornitz DM . (2004). Evolution of the Fgf and Fgfr gene families. Trends Genet 20: 563–569.
Jautelat R, Brumby T, Schafer M, Briem H, Eisenbrand G, Schwahn S et al. (2005). From the insoluble dye indirubin towards highly active, soluble CDK2-inhibitors. Chembiochem 6: 531–540.
Kang YJ, Seit-Nebi A, Davis RJ, Han J . (2006). Multiple activation mechanisms of p38alpha mitogen-activated protein kinase. J Biol Chem 281: 26225–26234.
Keenan SM, Bellone C, Baldassare JJ . (2001). Cyclin-dependent kinase 2 nucleocytoplasmic translocation is regulated by extracellular regulated kinase. J Biol Chem 276: 22404–22409.
Kosmopoulou MN, Leonidas DD, Chrysina ED, Bischler N, Eisenbrand G, Sakarellos CE et al. (2004). Binding of the potential antitumour agent indirubin-5-sulphonate at the inhibitor site of rabbit muscle glycogen phosphorylase b. Comparison with ligand binding to pCDK2-cyclin A complex. Eur J Biochem 271: 2280–2290.
Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL et al. (2001). Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem 276: 251–260.
Lents NH, Keenan SM, Bellone C, Baldassare JJ . (2002). Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J Biol Chem 277: 47469–47475.
Lin X, Buff EM, Perrimon N, Michelson AM . (1999). Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development 126: 3715–3723.
Losa JH, Parada CC, Viniegra JG, Sanchez-Arevalo LV, Cajal S, Sanchez-Prieto R . (2003). Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene 22: 3998–4006.
Lundberg AS, Weinberg RA . (1998). Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 18: 753–761.
Macdonald D, Aguiar RC, Mason PJ, Goldman JM, Cross NC . (1995). A new myeloproliferative disorder associated with chromosomal translocations involving 8p11: a review. Leukemia 9: 1628–1630.
Marko D, Schatzle S, Friedel A, Genzlinger A, Zankl H, Meijer L et al. (2001). Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br J Cancer 84: 283–289.
Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK et al. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276: 955–960.
Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S et al. (2005). Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA 102: 5998–6003.
Nguyen DT, Hernandez-Montes E, Vauzour D, Schonthal AH, Rice-Evans C, Cadenas E et al. (2006). The intracellular genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone mediates G2-M cell cycle arrest in cancer cells via modulation of the p38 signaling pathway. Free Radic Biol Med 41: 1225–1239.
Ornitz DM . (2000). FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22: 108–112.
Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A et al. (2004). Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem 47: 935–946.
Popovici C, Zhang B, Gregoire MJ, Jonveaux P, Lafage-Pochitaloff M, Birnbaum D et al. (1999). The t(6;8)(q27;p11) translocation in a stem cell myeloproliferative disorder fuses a novel gene, FOP, to fibroblast growth factor receptor 1. Blood 93: 1381–1389.
Powers CJ, McLeskey SW, Wellstein A . (2000). Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7: 165–197.
Ribas J, Bettayeb K, Ferandin Y, Knockaert M, Garrofe-Ochoa X, Totzke F et al. (2006). 7-Bromoindirubin-3′-oxime induces caspase-independent cell death. Oncogene 25: 6304–6318.
Roux PP, Blenis J . (2004). ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344.
Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS . (2000). A role for the p38 mitogen-activated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res 60: 2464–2472.
Sandvig K, Olsnes S . (1982). Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem 257: 7504–7513.
Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB . (2006). Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappaB signaling pathway. J Biol Chem 281: 23425–23435.
Tibbles LA, Woodgett JR . (1999). The stress-activated protein kinase pathways. Cell Mol Life Sci 55: 1230–1254.
Wiedlocha A, Falnes PO, Madshus IH, Sandvig K, Olsnes S . (1994). Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell 76: 1039–1051.
Xie Y, Liu Y, Ma C, Yuan Z, Wang W, Zhu Z et al. (2004). Indirubin-3′-oxime inhibits c-Jun NH2-terminal kinase: anti-apoptotic effect in cerebellar granule neurons. Neurosci Lett 367: 355–359.
Yu ZP, Matsuoka M, Wispriyono B, Iryo Y, Igisu H . (2000). Activation of mitogen-activated protein kinases by tributyltin in CCRF-CEM cells: role of intracellular Ca(2+). Toxicol Appl Pharmacol 168: 200–207.
Zhan X, Hu X, Friesel R, Maciag T . (1993). Long term growth factor exposure and differential tyrosine phosphorylation are required for DNA synthesis in BALB/c 3T3 cells. J Biol Chem 268: 9611–9620.
Acknowledgements
YZ is a PhD student at the University of Oslo, Faculty of Medicine, supported by the Norwegian Cancer Society. The skilful assistance with the cell cultures of Anne Engen and Anne Berit Dyve is gratefully acknowledged. This work was supported by grant DNK, T-974651001 from the Norwegian Cancer Society. We are grateful to Professors Jahn Nesland and Sjur Olsnes for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhen, Y., Sørensen, V., Jin, Y. et al. Indirubin-3′-monoxime inhibits autophosphorylation of FGFR1 and stimulates ERK1/2 activity via p38 MAPK. Oncogene 26, 6372–6385 (2007). https://doi.org/10.1038/sj.onc.1210473
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210473
Keywords
This article is cited by
-
Characterization of anti-leukemia components from Indigo naturalis using comprehensive two-dimensional K562/cell membrane chromatography and in silico target identification
Scientific Reports (2016)
-
The role of natural indigo dye in alleviation of genotoxicity of sodium dithionite as a reducing agent
Cytotechnology (2016)
-
Antimicrobial, antimycobacterial and antibiofilm properties of Couroupita guianensis Aubl. fruit extract
BMC Complementary and Alternative Medicine (2012)
-
Effect of Small Molecule Supplements during In Vitro Culture of Mouse Zygotes and Parthenogenetic Embryos on Hypoblast Formation and Stem Cell Derivation
Stem Cell Reviews and Reports (2012)
-
The ground state of embryonic stem cell self-renewal
Nature (2008)