Abstract
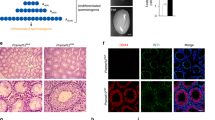
Sperm-associated antigen 1 (SPAG1) was recently identified in a rare form of infertility where anti-SPAG1 antibodies derived from the serum of an infertile woman were reported to cause sperm agglutination. Except for its expression and potential role in spermatogenesis, the function of SPAG1 is completely unknown. The unexpected finding of high levels of SPAG1 expression in pancreatic adenocarcinoma compared to normal pancreatic tissue in our previous cDNA array experiments prompted us to look in more detail at the expression and role of this gene in a panel of normal and malignant human tissues as well as in a larger series of pancreatic cancer specimens. We have generated an SPAG1-specific monoclonal antibody and showed high levels of SPAG1 protein in testis and in a large proportion of pancreatic ductal adenocarcinomas (PDAC). In the latter, SPAG1 expression was predominantly cytoplasmic and confined to malignant cells. Furthermore, the extent and intensity of SPAG1 expression was shown to be associated with stage and tumour nodal status, while analysis of precursor lesions, pancreatic intraepithelial neoplasias (PanINs), demonstrated its increased immunoreactivity with increasing PanIN grade, suggesting that SPAG1 is a novel marker of PDAC progression. Immunocytochemical analysis demonstrated colocalization of SPAG1 with microtubules, and their association was confirmed by co-immunoprecipitation; subsequent motility assays further substantiated a potential role of SPAG1 in cancer cell motility. Combined with the finding of its early expression in PDAC development, our data suggest that SPAG1 could contribute to the early spread and poor prognosis of pancreatic adenocarcinoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Accession codes
Abbreviations
- SPAG1:
-
sperm-associated antigen 1
- PanIN:
-
pancreatic intraepithelial neoplasia
- PDAC:
-
pancreatic ductal adenocarcinoma
- CP:
-
chronic pancreatitis
- TPR motif:
-
tetratricopeptide motif
- QRT–PCR:
-
quantitative real-time reverse transcription–polymerase chain reaction
- ESI, electrospray ionization:
-
CT genes' cancer/testis genes
- IHC:
-
immunohistochemistry
References
Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I et al. (2002). 5-aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin Cancer Res 8: 2690–2695.
Crnogorac-Jurcevic T, Missiaglia E, Blaveri E, Gangeswaran R, Jones M, Terris B et al. (2003). Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol 201: 63–74.
Cutillas PR, Chalkley RJ, Hansen KC, Cramer R, Norden AGW, Waterfield MD et al. (2004). The urinary proteome in Fanconi syndrome implies specificity in the reabsorption of proteins by renal proximal tubule cells. Am J Physiol 287: F353–F364.
Feinberg AP, Tycko B . (2004). The history of cancer epigenetics. Nat Rev Cancer 4: 143–153.
Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS . (1996). Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol 148: 1763–1770.
Gesierich S, Paret C, Hildebrand D, Weitz J, Zgraggen K, Schmitz-Winnenthal FH et al. (2005). Colocalization of the tetraspanins, CO-029 and CD151, with integrins in human pancreatic adenocarcinoma: impact on cell motility. Clin Cancer Res 11: 2840–2852.
Giehl K, Skripczynski B, Mansard A, Menke A, Gierschik P . (2000). Growth factor-dependent activation of the Ras-Raf-MEK-MAPK pathway in the human pancreatic carcinoma cell line PANC-1 carrying activated K-ras: implications for cell proliferation and cell migration. Oncogene 19: 2930–2942.
Grutzmann R, Boriss H, Ammerpohl O, Luttges J, Kalthoff H, Schackert HK et al. (2005). Meta-analysis of microarray data on pancreatic cancer defines a set of commonly dysregulated genes. Oncogene 24: 5079–5088.
Hustinx SR, Cao D, Maitra A, Sato N, Martin ST, Sudhir D et al. (2004). Differentially expressed genes in pancreatic ductal adenocarcinomas identified through serial analysis of gene expression. Cancer Biol Ther 3: 1254–1261.
Jemal A, Thomas A, Murray T, Thun M . (2002). Cancer statistics, 2002. CA Cancer J Clin 52: 23–47.
Kalejs M, Erenpreisa J . (2005). Cancer/testis antigens and gametogenesis: a review and ‘brain-storming’ session. Cancer Cell Int 5: 4.
Kubuschok B, Xie X, Jesnowski R, Preuss KD, Romeike BF, Neumann F et al. (2004). Expression of cancer testis antigens in pancreatic carcinoma cell lines, pancreatic adenocarcinoma and chronic pancreatitis. Int J Cancer 109: 568–575.
Lin W, Zhou X, Zhang M, Li Y, Miao S, Wang L et al. (2001). Expression and function of the HSD-3.8 gene encoding a testis-specific protein. Mol Hum Reprod 7: 811–818.
Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ et al. (2003). Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res 63: 2649–2657.
Michl P, Ramjaun AR, Pardo OE, Warne PH, Wagner M, Poulsom R et al. (2005). CUTL1 is a target of TGF(beta) signaling that enhances cancer cell motility and invasiveness. Cancer Cell 7: 521–532.
Missiaglia E, Blaveri E, Terris B, Wang YH, Costello E, Neoptolemos JP et al. (2004). Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer 112: 100–112.
Missiaglia E, Donadelli M, Palmieri M, Crnogorac-Jurcevic T, Scarpa A, Lemoine NR . (2005). Growth delay of human pancreatic cancer cells by methylase inhibitor 5-aza-2′-deoxycytidine treatment is associated with activation of the interferon signalling pathway. Oncogene 24: 199–211.
Old LJ . (2001). Cancer/testis (CT) antigens – a new link between gametogenesis and cancer. Cancer Immun 1: 1.
Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H et al. (2005). Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer 4: 37.
Scanlan MJ, Simpson AJ, Old LJ . (2004). The cancer/testis genes: review, standardization, and commentary. Cancer Immun 4: 1.
Shevchenko A, Wilm M, Vorm O, Mann M . (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858.
Sigalotti L, Coral S, Altomonte M, Natali L, Gaudino G, Cacciotti P et al. (2002). Cancer testis antigens expression in mesothelioma: role of DNA methylation and bioimmunotherapeutic implications. Br J Cancer 86: 979–982.
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ . (2005). Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615–625.
Small JV, Geiger B, Kaverina I, Bershadsky A . (2002). How do microtubules guide migrating cells? Nat Rev Mol Cell Biol 3: 957–964.
Takimoto M, Wei G, Dosaka-Akita H, Mao P, Kondo S, Sakuragi N et al. (2002). Frequent expression of new cancer/testis gene D40/AF15q14 in lung cancers of smokers. Br J Cancer 86: 1757–1762.
Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H et al. (2005). Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res 65: 3092–3099.
Tureci O, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M . (1998). Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci USA 95: 5211–5216.
Vasiliev JM . (2004). Cytoskeletal mechanisms responsible for invasive migration of neoplastic cells. Int J Dev Biol 48: 425–439.
Zhang M, Zhou X, Cao D, Miao S, Wang L . (1999). Tissue specificity and expression of a human sperm protein's gene BSD-2.4. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 21: 166–169.
Zhang ML, Wang LF, Miao SY, Koide SS . (1992). Isolation and sequencing of the cDNA encoding the 75-kD human sperm protein related to infertility. Chin Med J (Engl) 105: 998–1003.
Acknowledgements
We thank Dr David Snary (Applied Development Laboratory, CRT) for assistance in selecting the peptide epitopes and Ms Maggie Stubbs for generating monoclonal SPAG1 antibody; Dr Wang (Institute of Basic Medical Sciences, Beijing, China) for anti-SPAG1 polyclonal antibody; Professor Holger Kalthoff (University of Kiel, Germany) for serum samples; Dr Kit-Yi Leung (Clinical Pharmacology) for help with MS and Ms Catherine Okello (Thames Cancer Registry) for providing the missing patient information. This work was supported by Cancer Research UK, Mike Stone Cancer Research Fund and Graduate College 460 grant, University of Ulm, Germany (AN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Supplementary information
Rights and permissions
About this article
Cite this article
Neesse, A., Gangeswaran, R., Luettges, J. et al. Sperm-associated antigen 1 is expressed early in pancreatic tumorigenesis and promotes motility of cancer cells. Oncogene 26, 1533–1545 (2007). https://doi.org/10.1038/sj.onc.1209961
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209961
Keywords
This article is cited by
-
Intracystic papillary carcinoma of breast: interrelationship with in situ and invasive carcinoma and a proposal of pathogenesis: array comparative genomic hybridization study of 14 cases
Modern Pathology (2014)
-
Down regulation of SPAG9 reduces growth and invasive potential of triple-negative breast cancer cells: possible implications in targeted therapy
Journal of Experimental & Clinical Cancer Research (2013)
-
S100P is a metastasis-associated gene that facilitates transendothelial migration of pancreatic cancer cells
Clinical & Experimental Metastasis (2013)