Abstract
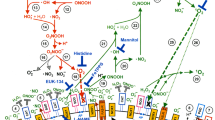
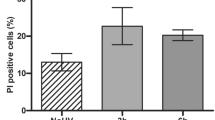
Downregulation of survival signaling pathways contributes to the cytotoxicity of reactive oxygen species (ROS) and may underlie certain therapies for hyperproliferative diseases. We have investigated the role of singlet oxygen, an ROS formed by photosensitization, in the regulation of survival signaling via the epidermal growth factor receptor (EGFR). Exposure of human keratinocytes to singlet oxygen resulted in rapid loss of EGFR, which was not blocked by either inhibition of receptor internalization or by interrupting the major proteolytic pathways (proteasome, lysosome or calpain). However, pretreatment with a caspase-3 inhibitor, DEVD-FMK, inhibited EGFR degradation. Caspase-3 cleavage was detected as early as 5 min after singlet oxygen treatment, and recombinant active caspase-3 completely cleaved EGFR in a keratinocyte membrane fraction. The singlet oxygen-induced loss of EGFR was accompanied by dephosphorylation of EGFR as well as of Akt and extracellular signal-regulated kinase 1/2 (ERK)1/2. Singlet oxygen-induced protein dephosphorylation was not dependent on activation of caspase-3. In contrast, inhibition of protein phosphatases (PPs) with okadaic acid completely blocked dephosphorylation of EGFR, ERK1/2 and Akt as well as degradation of EGFR. These results indicate that the oxidative stress produced by singlet oxygen rapidly disrupts EGFR-mediated signaling by decreasing both the protein level and its phosphorylation. These responses depended on intertwined activation of caspase-3 and PPs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- EGFR:
-
epidermal growth factor receptor
- ERK:
-
extracellular signal-regulated kinase
- OA:
-
okadaic acid
- PP:
-
protein phosphatase
- PTP:
-
protein tyrosine phosphatase
- PUVA:
-
psoralen/UVA
- RB:
-
Rose Bengal
- ROS:
-
reactive oxygen species
References
Ahmad N, Kalka K and Mukhtar H . (2001). Oncogene, 20, 2314–2317.
An B, Jin JR, Lin P and Dou QP . (1996). FEBS Lett., 399, 158–162.
Authier F, Metioui M, Bell AW and Mort JS . (1999). J. Biol. Chem., 274, 33723–33731.
Bae SS, Choi JH, Oh YS, Perry DK, Ryu SH and Suh PG . (2001). FEBS Lett., 491, 16–20.
Baker A and Kanofsky JR . (1992). Photochem. Photobiol., 55, 523–528.
Bar-Sagi D, Suhan JP, McCormick F and Feramisco JR . (1988). J. Cell. Biol., 106, 1649–1658.
Boulougouris P and Elder J . (2001). Anticancer Res., 21, 2769–2775.
Buttle DJ, Murata M, Knight CG and Barrett AJ . (1992). Arch. Biochem. Biophys., 299, 377–380.
Cayla X, Goris J, Hermann J, Hendrix P, Ozon R and Merlevede W . (1990). Biochemistry, 29, 658–667.
Cayla X, Van Hoof C, Bosch M, Waelkens E, Vandekerckhove J, Peeters B, Merlevede W and Goris J . (1994). J. Biol. Chem., 269, 15668–15675.
Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A and Hannun YA . (1999). J. Biol. Chem., 274, 20313–20317.
Davies PJ, Davies DR, Levitzki A, Maxfield FR, Milhaud P, Willingham MC and Pastan IH . (1980). Nature, 283, 162–167.
Denu JM and Tanner KG . (1998). Biochemistry, 37, 5633–5642.
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP and Rheinwald JG . (2000). Mol. Cell. Biol., 20, 1436–1447.
Dou QP, An B and Will PL . (1995). Proc. Natl. Acad. Sci. USA, 92, 9019–9023.
Fladmark KE, Brustugun OT, Hovland R, Boe R, Gjertsen BT, Zhivotovsky B and Doskeland SO . (1999). Cell Death Differ., 6, 1099–1108.
Flint AJ, Tiganis T, Barford D and Tonks NK . (1997). Proc. Natl. Acad. Sci. USA, 94, 1680–1685.
Gregoriou M, Willis AC, Pearson MA and Crawford C . (1994). Eur. J. Biochem., 223, 455–464.
Grether-Beck S, Bonizzi G, Schmitt-Brenden H, Felsner I, Timmer A, Sies H, Johnson JP, Piette J and Krutmann J . (2000). EMBO J., 19, 5793–5800.
Hunter I, McGregor D and Robins SP . (2001). J. Bone Miner. Res., 16, 466–477.
Janssens V and Goris J . (2001). Biochem. J., 353, 417–439.
Kamata H, Shibukawa Y, Oka SI and Hirata H . (2000). Eur. J. Biochem., 267, 1933–1944.
Katoh S . (1994). Biol. Pharm. Bull., 17, 1003–1107.
Kessel D and Castelli M . (2001). Photochem. Photobiol., 74, 318–322.
Kidd VJ, Lahti JM and Teitz T . (2000). Semin. Cell Dev. Biol., 11, 191–201.
Kishikawa K, Chalfant CE, Perry DK, Bielawska A and Hannun YA . (1999). J. Biol. Chem., 274, 21335–21341.
Kolch W . (2000). Biochem. J., 351, 289–305.
Krueger JG, Krane JF, Carter DM and Gottlieb AB . (1990). J. Invest. Dermatol., 94, 135S–140S.
Laskin JD, Lee E, Laskin DL and Gallo MA . (1986). Proc. Natl. Acad. Sci. USA, 83, 8211–8215.
Law B and Rossie S . (1995). J. Biol. Chem., 270, 12808–12813.
Lawlor MA and Alessi DR . (2001). J. Cell. Sci., 114, 2903–2910.
Lee SR, Kwon KS, Kim SR and Rhee SG . (1998). J. Biol. Chem., 273, 15366–15372.
Li DW, Xiang H, Mao YW, Wang J, Fass U, Zhang XY and Xu C . (2001). Exp. Cell Res., 266, 279–291.
Lin CP, Lynch MC and Kochevar IE . (2000). Exp. Cell Res., 259, 351–359.
Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE and Madshus IH . (2002). J. Cell Biol., 156, 843–854.
MacKintosh C and MacKintosh RW . (1994). Trends Biochem. Sci., 19, 444–448.
Maziere C, Conte MA, Leborgne L, Levade T, Hornebeck W, Santus R and Maziere JC . (2001). Biochem. Biophys. Res. Commun., 281, 289–294.
Mendelsohn J and Baselga J . (2000). Oncogene, 19, 6550–6565.
Millward TA, Zolnierowicz S and Hemmings BA . (1999). Trends Biochem. Sci., 24, 186–191.
Morita A, Werfel T, Stege H, Ahrens C, Karmann K, Grewe M, Grether-Beck S, Ruzicka T, Kapp A, Klotz LO, Sies H and Krutmann J . (1997). J. Exp. Med., 186, 1763–1768.
Nicholson DW . (1999). Cell Death Differ., 6, 1028–1042.
Oleinick NL and Evans HH . (1998). Radiat. Res., 150, S146–S156.
Posmantur R, McGinnis K, Nadimpalli R, Gilbertsen RB and Wang KK . (1997). J. Neurochem., 68, 2328–2337.
Ravid T, Sweeney C, Gee P, Carraway III KL and Goldkorn T . (2002). J. Biol. Chem., 277, 31214–31219.
Rosette C and Karin M . (1996). Science, 274, 1194–1197.
Santoro MF, Annand RR, Robertson MM, Peng YW, Brady MJ, Mankovich JA, Hackett MC, Ghayur T, Walter G, Wong WW and Giegel DA . (1998). J. Biol. Chem., 273, 13119–13128.
Scheving LA, Jin WH, Chong KM, Gardner W and Cope FO . (1998). Am. J. Physiol., 274, C1363–C1372.
Schutze S . (1999). J. Biol. Chem., 274, 10203–10212.
Separovic D, Mann KJ and Oleinick NL . (1998). Photochem. Photobiol., 68, 101–109.
Silberman SR, Speth M, Nemani R, Ganapathi MK, Dombradi V, Paris H and Lee EY . (1984). J. Biol. Chem., 259, 2913–2922.
Song Q and Lavin MF . (1993). Biochem. Biophys. Res. Commun., 190, 47–55.
Spizz G and Blackshear PJ . (1997). J. Biol. Chem., 272, 23833–23842.
Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K and Huber O . (2001). J. Biol. Chem., 276, 4972–4980.
Vanderklish PW and Bahr BA . (2000). Int. J. Exp. Pathol., 81, 323–339.
van der Vliet A, Hristova M, Cross CE, Eiserich JP and Goldkorn T . (1998). J. Biol. Chem., 273, 31860–31866.
Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM and Hannun YA . (1994). J. Biol. Chem., 269, 19605–19609.
Yarden Y . (2001). Eur. J. Cancer, 37, S3–S8.
Yoshimori T, Yamamoto A, Moriyama Y, Futai M and Tashiro Y . (1991). J. Biol. Chem., 266, 17707–17712.
Zhuang S, Demirs JT and Kochevar IE . (2000). J. Biol. Chem., 275, 25939–25948.
Zhuang S, Lynch MC and Kochevar IE . (1999). Exp. Cell Res., 250, 203–212.
Acknowledgements
We thank Dr James G Rheinwald and the Harvard Skin Disease Research Center for providing normal human TERT keratinocytes. This work was supported by NIH Grant GM 30755.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuang, S., Ouedraogo, G. & Kochevar, I. Downregulation of epidermal growth factor receptor signaling by singlet oxygen through activation of caspase-3 and protein phosphatases. Oncogene 22, 4413–4424 (2003). https://doi.org/10.1038/sj.onc.1206604
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1206604
Keywords
This article is cited by
-
Interaction of EGFR to δ-catenin leads to δ-catenin phosphorylation and enhances EGFR signaling
Scientific Reports (2016)
-
cis-Urocanic Acid Enhances Prostaglandin E2 Release and Apoptotic Cell Death via Reactive Oxygen Species in Human Keratinocytes
Journal of Investigative Dermatology (2011)
-
Proteolytic cleavages give receptor tyrosine kinases the gift of ubiquity
Oncogene (2009)
-
Selective apoptosis induction by the cancer chemopreventive agent N-(4-hydroxyphenyl)retinamide is achieved by modulating mitochondrial bioenergetics in premalignant and malignant human prostate epithelial cells
Apoptosis (2009)
-
Role of epidermal growth factor receptor degradation in gemcitabine-mediated cytotoxicity
Oncogene (2007)