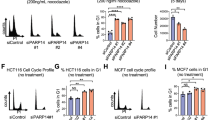
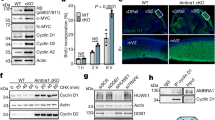
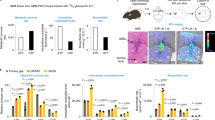
Abstract
The human Ink4a/Arf tumor suppressor locus encodes two distinct products: p16Ink4a which prevents phosphorylation and inactivation of the retinoblastoma protein and, p14Arf, a nucleolar protein which activates the function of the tumor suppressor p53 protein in the nucleoplasm in response to oncogenic stimulation through an as yet ill-defined mechanism. Here we show that the level of endogenous p14Arf and its balance between the nucleolus and the nucleoplasm in HeLa cells are exquisitely sensitive to changes in cell morphology and to short-lived perturbations in cell cycle and in nucleolar function such as those induced by the cyclin-dependent kinase inhibitor, roscovitine, and the casein kinase II and RNA synthesis inhibitor, DRB. Most remarkably, whereas p14Arf predominantly concentrates in the nucleolus of interphase cells and transiently disappears between metaphase and early G1 under normal growth conditions, it massively and reversibly accumulates in the nucleoplasm of postmitotic and S-phase cells upon short-term treatment with roscovitine and, at a lesser extent, DRB. In line with the fact that the nuclear level of p53 reaches a peak between mid-G1 and the G1/S border in p53-expressor cells which lack Arf expression, these results provide a clue that, in p53+/Arf+ cells, Arf proteins might serve both to speed and to amplify p53-mediated responses in conditions and cell cycle periods in which the mechanisms involved in p53 stabilization and activation are not fully operational. They further suggest that human endogenous p14Arf might activate p53 pathways in physiologic situations by acting inside the nucleoplasm, especially when normal cell cycle progression and nucleolar function are compromised.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- Cdk:
-
cyclin-dependent kinase
- CKII:
-
casein kinase II
- DRB:
-
5,6-dichloro-1-β-D-ribofuranosylbenzimidazole
- BrdUrd:
-
5-bromodeoxyuridine
- Dapi:
-
4′,6-diamidino-2-phenylindole
- MEFs:
-
mouse embryo fibroblasts
- NDFs:
-
nucleolus-derived foci
- PNBs:
-
prenucleolar bodies
- DMEM:
-
Dulbecco's modified Eagle's medium
- IPTG:
-
isopropyl β-D-thiogalactopyranoside
- FITC:
-
fluorescein isothiocyanate
- TRITC:
-
tetramethyl rhodamine isothiocyanate
References
Agarwal ML, Taylor WR, Chernov MW, Chernova OB, Stark GR . 1998 J. Biol. Chem. 273: 1–4
Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH . 1998 Nature 395: 124–125
Bosselut R, Duvall JF, Gégonne A, Bailly M, Hémar A, Brady J, Ghysdael J . 1990 EMBO J. 9: 22–27
Cong F, Zou X, Hinrichs K, Calame K, Goff SP . 1999 Oncogene 18: 7731–7739
David-Pfeuty T, Chakrani F, Ory K, Nouvian-Dooghe Y . 1996 Cell Growth Diff. 7: 1211–1225
David-Pfeuty T . 1999 Oncogene 18: 7409–7422
David-Pfeuty T, Nouvian-Dooghe Y, Sirri V, Roussel P, Hernandez-Verdun D . 2001 Oncogene 20: 5951–5963
DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR . 1997 Proc. Natl. Acad. Sci. USA 94: 7245–7250
Della Valle V, Duro D, Bernard O, Larsen C-J . 1997 Oncogene 15: 2475–2481
de Stanchina E, McCurrach ME, Zindy F, Shieh S-Y, Ferbeyre G, Samuelson AV, Prives C, Roussel MF, Sherr CJ, Lowe SW . 1998 Genes Dev. 12: 2434–2442
Dimri GP, Itahana K, Acosta M, Campisi J . 2000 Mol. Cell. Biol. 20: 273–285
Dundr M, Olson MOJ . 1998 Mol. Biol. Cell 9: 2407–2422
Dundr M, Misteli T, Olson MOJ . 2000 J. Cell. Biol. 150: 433–446
Duro D, Bernard O, Della Valle V, Berger R, Larsen C-J . 1995 Oncogene 11: 21–29
Eymin B, Karayan L, Séité P, Brambilla C, Bambrilla E, Larsen C-J, Gazzéri S . 2001 Oncogene 20: 1033–1041
Gautier T, Robert-Nicoud M, Guilly M-N, Hernandez-Verdun D . 1992 J. Cell. Sci. 102: 729–737
Giaccia AJ, Kastan MB . 1998 Genes Dev. 12: 2973–2983
Girard F, Srausfeld U, Fernandez A, Lamb N . 1991 Cell 67: 1169–1179
Goodwin EC, DiMaio D . 2000 Proc. Natl. Acad. Sci. USA 97: 12513–12518
Granick D . 1975 J. Cell. Biol. 65: 418–427
Haaf T, Ward DC . 1996 Exp. Cell. Res. 224: 163–173
Harbour JW, Dean DC . 2000 Genes Dev. 14: 2393–2409
Hartwell L . 1992 Cell 71: 543–546
Hartwell L, Weinert TA . 1989 Science 246: 629–634
Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris CC . 1994 Nucleic Acids Res. 22: 3552–3555
Honda R, Yasuda H . 1999 EMBO J. 18: 22–27
Kamijo T, Weber JD, Zambetti G, Zindy F, Roussel MF, Sherr CJ . 1998 Proc. Natl. Acad. Sci. USA 95: 8292–8297
Karayan L, Riou J-F, Séité P, Migeon J, Cantereau A, Larsen C-J . 2001 Oncogene 20: 836–848
Khan SH, Wahl GM . 1998 Cancer Res. 58: 396–401
Khan SH, Moritsugu J, Wahl GM . 2000 Proc. Natl. Acad. Sci. USA 97: 3266–3271
Ko LJ, Prives C . 1996 Genes Dev. 10: 1054–1072
Llanos S, Clark PA, Rowe J, Peters G . 2001 Nat. Cell. Biol. 3: 445–452
Levine AJ . 1997 Cell 88: 323–331
Lin AW, Lowe SW . 2001 Proc. Natl. Acad. Sci. USA 98: 5025–5030
Lindström MS, Klangby U, Inoue R, Pisa P, Wiman KG, Asker CE . 2000 Exp. Cell. Res. 256: 400–410
Lohrum MAE, Ashcroft M, Kubbutat MHG, Vousden KH . 2000 Curr. Biol. 10: 539–542
Lundberg AS, Hahn WC, Gupta P, Weinberg RA . 2000 Curr. Opin. Cell. Biol. 12: 705–709
Mao L, Merlo A, Bedi G, Shapiro GI, Edwards CD, Rollins BJ, Sidransky D . 1995 Cancer Res. 55: 2995–2997
Meijer L, Borgne A, Mulner O, Chong JPJ, Blow JJ, Inagaki N, Inagaki M, Delcros J-G, Moulinoux J-P . 1997 Eur. J. Biochem. 243: 527–536
Morgan DO . 1999 Nat. Cell. Biol. 1: 47–53
Nevins JR . 2000 Hum. Mol. Gen. 10: 699–703
O'Connor PM . 1997 Cancer Surveys 29: 151–181
Palmero I, Pantoja C, Serrano M . 1998 Nature 395: 125–126
Pardee AB . 1974 Proc. Natl. Acad. Sci. USA 71: 1286–1290
Pomerantz J, Shreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H-W, Cordon-Carlo C, DePinho RA . 1998 Cell 92: 713–723
Prives C . 1998 Cell 95: 5–8
Quelle DE, Zindy F, Ashmun RA, Sherr CJ . 1995 Cell 83: 993–1000
Radfar A, Unnikrishnan I, Lee H-W, DePinho RA, Rosenberg N . 1998 Proc. Natl. Acad. Sci. USA 95: 13194–13199
Savino TM, Gébrane-Younès J, De Mey J, Sibarita J-B, Hernandez-Verdun D . 2001 J. Cell. Biol. 153: 1097–1110
Savino TM, Bastos R, Jansen E, Hernandez-Verdun D . 1999 J. Cell. Sci. 112: 1889–1900
Sharpless NE, DePinho RA . 1999 Curr. Opin. Gen. Dev. 9: 22–30
Sherr CJ . 1998 Genes Dev. 12: 2984–2991
Sherr CJ, Weber JD . 2000 Curr. Opin. Gen. Dev. 10: 94–99
Sirri V, Hernandez-Verdun D, Roussel P . 2002 J. Cell Biol. 156: 968–981
Sirri V, Roussel P, Hernandez-Verdun D . 2000 J. Cell. Biol. 148: 259–270
Stone S, Jiang P, Dayananth P, Tavtigian SV, Katcher H, Parry D, Peters G, Kamb A . 1995 Cancer Res. 55: 2988–2994
Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH, Peters G . 1998 EMBO J. 17: 5001–5014
Takahashi K, Suzuki K . 1994 Oncogene 9: 183–188
Tao W, Levine AJ . 1999 Proc. Natl. Acad. Sci. USA 96: 6937–6941
Vidal A, Koff A . 2000 Gene 247: 1–15
Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D . 1999 Nat. Cell. Biol. 1: 20–26
Weber JD, Kuo ML, Bothner B, DiGiammarino EL, Kriwacki RW, Roussel MF, Sherr CJ . 2000 Mol. Cell. Biol. 20: 2517–2528
Wilson GD, McNally NJ, Dunphy E, Kärcher H, Pfragner R . 1985 Cytometry 6: 641–647
Wyllie AH, Kerr JFR, Currie AR . 1980 Int. Rev. Cytol. 68: 251–306
Zhang Y, Xiong Y . 1999 Mol. Cell 3: 579–591
Zhang Y, Xiong Y, Yarbrough WG . 1998 Cell 92: 725–734
Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, Roussel MF . 1998 Genes Dev. 12: 2424–2433
Acknowledgements
We are greatly indebted to Gordon Peters for his generosity in providing us with the 4c6/4 mouse monoclonal and JR14 rabbit polyclonal anti-p14Arf antibodies as well as with the NARF2 and NARF2/E6 cell lines and to Susana Llanos, for her valuable information and advices. We are thankful also to Tony Hunter for supplying the anti-cyclin A antiserum. Our acknowledgements extend to Frederic Coquelle for teaching us microscope imaging and to Dany Rouillard for her help with the flow cytometry experiments. The study was supported by the Centre National pour la Recherche Scientifique and the Curie Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
David-Pfeuty, T., Nouvian-Dooghe, Y. Human p14Arf: an exquisite sensor of morphological changes and of short-lived perturbations in cell cycle and in nucleolar function. Oncogene 21, 6779–6790 (2002). https://doi.org/10.1038/sj.onc.1205871
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205871
Keywords
This article is cited by
-
DNA damage, p14ARF, Nucleophosmin (NPM/B23), and cancer
Journal of Molecular Histology (2006)
-
The moving parts of the nucleolus
Histochemistry and Cell Biology (2005)
-
Stability of nucleolar versus non-nucleolar forms of human p14ARF
Oncogene (2004)