Abstract
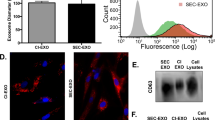
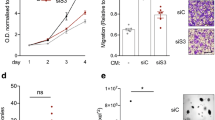
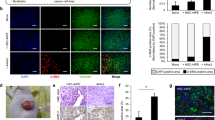
Metalloproteinases (MMP) produced by both cancer and normal stromal fibroblast cells play a critical role in the metastatic spread of tumours, however little is known of the regulation of their release. In this report we demonstrate that breast cancer cells in culture release apparently full length soluble EMMPRIN that promotes the release of pro-MMP2 from fibroblasts. The generation of MMP2 is mediated by activation of phospholipase A2 and 5-lipoxygenase. These results suggest that the production of soluble EMMPRIN, phospholipase A2 and 5-lipoxygenase activities are sites for potential therapeutic intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Balsinde J, Dennis EA . 1996 J. Biol. Chem. 271: 6758–6765
Bassett P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P . 1990 Nature 348: 699–704
Berditchevski F, Chang S, Bodorova J, Hemler ME . 1997 J. Biol. Chem. 272: 29174–29180
Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K . 1995 Cancer Res. 55: 434–439
Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d'Ortho MP, Murphy G . 1998 J. Biol. Chem. 273: 871–880
Caudroy S, Polette M, Tournier JM, Burlett H, Toole B, Zucker B, Birembaut P . 1999 J. Histochem. Cytochem. 47: 1575–1580
D'Errico A, Garbisa S, Liotta LA, Castronovo V, Stetler-Stevenson WG, Grigioni WF . 1991 Mod. Pathol. 4: 239–246
DeCastro R, Zhang Y, Guo HM, Kataoka H, Gordon MK, Toole BP, Biswas C . 1996 J. Invest. Dermatol. 106: 1260–1265
Ellis SM, Nabeshima K, Biswas C . 1989 Cancer Res. 49: 3385–3391
Ferry DR, Deakin M, Baddeley J, Daryanani S, Bramhall S, Anderson DA, Wakelam MJO, Doran J, Pemberton G, Young AM, Buckels J, Kerr DJ . 2000 Ann. Oncol. 11: 1165–1170
Guo H, Zucker S, Gordon MK, Bryan BP, Biswas C . 1997 J. Biol. Chem. 272: 24–27
Huang MT, Newmark HL, Frenkel K . 1997 J. Cell. Biochem. Suppl. 27 26–34
Kleiner DE, Stetler-Stevenson WG . 1999 Cancer Chemother. Pharmacol. 43: 42–51
Light DB, Mertins TM, Belongia JA, Witt CA . 1997 J. Membr. Biol. 158: 229–239
Lim M, Martinez T, Jablons D, Cameron R, Guo H, Toole B, Li J, Basbaum C . 1998 FEBS Letters 441: 88–92
Mohan R, Sivak J, Ashton P, Russo LA, Pham BQ, Kasahara N, Raizman MB, Fini ME . 2000 J. Biol. Chem. 275: 10405–10412
Nabeshima K, Lane WS, Biswas C . 1991 Arch. Biochem. Biophys 285: 90–96
Nomura H, Sato H, Seiki M, Mai M, Okada Y . 1995 Cancer Res. 55: 3263–3266
Sternlicht MD, Lochter A, Sympson CJ, Juey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z . 1999 Cell 98: 137–146
Sun JX, Hemler ME . 2001 Cancer Res. 61: 2276–2281
Ward RV, Atkinson SJ, Reynolds JJ, Murphy G . 1994 Biochem. J. 304: 263–269
Winstead MV, Balsinde J, Dennis EA . 2000 Biochim. Biophys. Acta 1488: 28–39
Zeng ZS, Cohen AM, Guillem JG . 1999 Carcinogenesis 20: 749–755
Zucker S, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J, Foda HD, Tompkins DC, Toole BP . 2001 Am. J. Pathol. 158: 1921–1928
Acknowledgements
This work was supported by Cancer Research UK and The Wellcome Trust, MN Hodgkin was a Beit Memorial Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, P., Woodfield, R., Hodgkin, M. et al. Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A2 and 5-lipoxygenase catalyzed pathway. Oncogene 21, 5765–5772 (2002). https://doi.org/10.1038/sj.onc.1205702
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205702
Keywords
This article is cited by
-
Crosstalk Between Matrix Metalloproteinases and Their Inducer EMMPRIN/CD147: a Promising Therapeutic Target for Intracerebral Hemorrhage
Translational Stroke Research (2023)
-
CD147 promotes collective invasion through cathepsin B in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2020)
-
Modulation of reactive oxygen levels and gene expression in sensitive and resistant tumoral cells by C-phyocyanin
Molecular Biology Reports (2019)
-
Combination of VP3 and CD147-knockdown enhance apoptosis and tumor growth delay index in colorectal tumor allograft
BMC Cancer (2016)
-
Functional role of EMMPRIN in the formation and mineralisation of dental matrix in mouse molars
Journal of Molecular Histology (2015)