Abstract
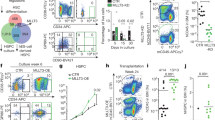
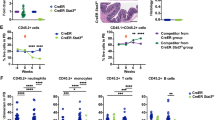
STAT3 is a key downstream signaling intermediate of gp130, a receptor previously shown to activate hematopoietic stem cell (HSC) self-renewal divisions. These findings prompted us to investigate if the STAT3 pathway is important to HSC activity in vivo. Initial semi-quantitative RT–PCR analyses showed STAT3 to be expressed at slightly higher levels in primitive subsets of both human and murine adult bone marrow cells. To test the effect of abrogating STAT3 activity in HSCs, primitive murine fetal liver cells were transduced at high efficiency with either a bicistronic dominant-negative (dn) or wild-type (wt) STAT3-IRES-GFP retrovirus. Dn STAT3-transduced HSCs showed markedly and permanently reduced in vivo lympho-myeloid reconstituting ability relative to co-transplanted non-transduced HSCs or HSCs transduced with a control (GFP-only) vector. In contrast, the activity of dn STAT3-transduced cells with short term in vivo (CFU-S) or in vitro (CFC) proliferation potential was not affected. Overexpression of wt-STAT3 had very little effect on either HSCs or shorter term progenitors. These findings suggest HSCs express non-limiting levels of STAT3 which, nevertheless, play an important stage-specific and non-redundant role in maintaining the function of HSCs stimulated to divide in adult marrow tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Audet J, Miller CL, Rose-John S, Piret J, Eaves CJ . 2001 Proc. Natl. Acad. Sci. USA 98: 1757–1762
Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JC . 1994 Immunity 1: 725–731
Biethahn S, Alves F, Wilde S, Hiddeman W, Spiekermann K . 1999 Exp. Hematol. 27: 885–894
Caldenhoven E, van Dijk TB, Solari R, Armstrong J, Raaijmakers JAM, Lammers W-WJ, Koenderman L, de Groot RP . 1996 J. Biol. Chem. 22: 13221–13227
Chakraborty A, Tweardy DJ . 1998 Leuk. Lymphoma 30: 433–442
Chakraborty A, White SM, Schaefer TS, Ball ED, Dyer KF, Tweardy DJ . 1996 Blood 88: 2442–2449
Cheng T, Rodrigues N, Shen H, Yang Y-G, Dombkowski D, Sykes M, Scadden DT . 2000 Science 287: 1804–1808
Conneally E, Cashman J, Petzer A, Eaves C . 1997 Proc. Natl. Acad. Sci. USA 94: 9836–9841
Darnell Jr JE . 1997 Science 277: 1630–1635
Fraser CC, Szilvassy SJ, Eaves CJ, Humphries RK . 1992 Proc. Natl. Acad. Sci. USA 89: 1968–1972
Hennemann B, Chuo JY, Schley PD, Lambie K, Humphries RK, Eaves CJ . 2000 Hum. Gene Ther. 11: 43–51
Holyoake T, Jiang X, Eaves C, Eaves A . 1999 Blood 94: 2056–2064
Kishimoto A, Akira S, Narazaki M, Taga T . 1995 Blood 86: 1243–1254
Lemieux ME, Rebel VI, Lansdorp PM, Eaves CJ . 1995 Blood 86: 1339–1347
Markowitz D, Goff S, Bank A . 1988 J. Virol. 62: 1120–1124
Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T . 1999 EMBO J. 18: 4261–4269
Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, Akira S . 1996 Proc. Natl. Acad. Sci. USA 93: 3963–3966
Niwa M, Burdon T, Chambers I, Smith A . 1998 Genes Dev. 12: 2048–2060
O'Farrell A-M, Liu Y, Moore KW, Mui AL-F . 1998 EMBO J. 17: 1006–1018
Oh I-H, Lau A, Eaves CJ . 2000 Blood 96: 4160–4168
Peters M, Schirmacher P, Goldschmitt J, Odenthal M, Peschel C, Dienes H-P, Fattori E, Ciliberto G, Meyer zum Buschenfelde KH, Rose-John S . 1997 J. Exp. Med. 185: 755–766
Petzer AL, Hogge DE, Lansdorp PM, Reid DS, Eaves CJ . 1996 Proc. Natl. Acad. Sci. USA 93: 1470–1474
Raz R, Lee CK, Cannizzarro LA, d'Eustachio P, Levy DE . 1999 Proc. Natl. Acad. Sci. USA 96: 2846–2851
Rebel VI, Lansdorp PM . 1996 J. Hematother. 5: 25–37
Rebel VI, Miller CL, Eaves CJ, Lansdorp PM . 1996 Blood 87: 3500–3507
Sasse J, Hemmann U, Schwartz C, Schniertshauer U, Heesel B, Landgraf C, Schneider-Mergener J, Heinrich P, Horn F . 1997 Mol. Cell. Biol. 17: 4677–4686
Schaefer TS, Sanders LK, Nathans D . 1995 Proc. Natl. Acad. Sci. USA 92: 9097–9101
Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ . 1990 Proc. Natl. Acad. Sci. USA 87: 8736–8740
Szilvassy SJ, Nicolini FE, Eaves CJ, Miller CL . 2001 Methods in Molecular Medicine: Hematopoietic Stem Cell Protocols: Quantitation of murine and human hematopoietic stem cells by limiting-dilution analysis in competitively repopulated hosts Jordon CT and Klug CA (eds) Totowa, NJ: Humana Press pp. 167–187
Taga T, Kishimoto T . 1997 Annu. Rev. Immunol. 15: 797–819
Thomas TE, Miller CL, Eaves CJ . 1999 Methods: A Companion to Methods in Enzymology: Purification of hematopoietic stem cells for further biological study Vol 17: New York: Academic Press pp 202–218
Wang JCY, Doedens M, Dick JE . 1997 Blood 89: 3919–3924
Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang W-Z, Mori C, Shiota K, Yoshida N, Kishimoto T . 1996 Proc. Natl. Acad. Sci. USA 93: 407–411
Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ . 1997 Proc. Natl. Acad. Sci. USA 94: 4698–4703
Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S . 1995 Science 267: 1990–1994
Zong CS, Zeng L, Jiang Y, Sadowski HB, Wang LH . 1998 J. Biol. Chem. 273: 28065–28072
Acknowledgements
The authors thank Cindy Cao, Geoff Gotto, Maya Sinclair, Margaret Hale, and the staff of the Terry Fox Laboratory FACS service for expert technical assistance, Dr A Mui for the STAT3 plasmids, Dr K Humphries for the MSCV–IRES–GFP plasmid and Amy Ahamed for typing. This study was supported by the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society and the Terry Fox Run. I-H Oh held a NCIC Postdoctoral Fellowship and C Eaves was a Terry Fox Cancer Research Scientist of the NCIC.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Inhibition of endogenous STAT3 activation by dn STAT3 in stably transduced M1 cells. Aliquots of cells stably-transduced with each retroviral vector were stimulated with IL-6 (100 ng/ml) for 15 min and then analysed by Western blotting for the presence of phosphorylated STAT3 using an anti-phosphotyrosine (Tyr705)-specific anti-STAT3 antibody (105 cells per lane). The membrane was then stripped and re-probed for total STAT3 protein using a polyclonal antibody against STAT3 (see Figure 10).

Figure 10
Rights and permissions
About this article
Cite this article
Oh, IH., Eaves, C. Overexpression of a dominant negative form of STAT3 selectively impairs hematopoietic stem cell activity. Oncogene 21, 4778–4787 (2002). https://doi.org/10.1038/sj.onc.1205592
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1205592
Keywords
This article is cited by
-
Acute myeloid leukemia cell-derived extracellular vesicles carrying microRNA-548ac regulate hematopoietic function via the TRIM28/STAT3 pathway
Cancer Gene Therapy (2022)
-
The Dual Role of ROS in Hematological Malignancies: Stem Cell Protection and Cancer Cell Metastasis
Stem Cell Reviews and Reports (2020)
-
Acute myeloid leukemia cells secrete microRNA-4532-containing exosomes to mediate normal hematopoiesis in hematopoietic stem cells by activating the LDOC1-dependent STAT3 signaling pathway
Stem Cell Research & Therapy (2019)
-
Hematopoietic Stem Cells in Neural-crest Derived Bone Marrow
Scientific Reports (2016)
-
Transient Tcf3 Gene Repression by TALE-Transcription Factor Targeting
Applied Biochemistry and Biotechnology (2016)