Abstract
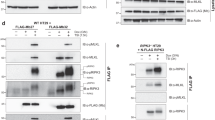
Meltrin α/ADAM12 is a member of the ADAM/MDC family proteins characterized by the presence of metalloprotease and disintegrin domains. This protein also contains a single transmembrane domain and a relatively long cytoplasmic domain containing several proline-rich sequences. These sequences are compatible with the consensus sequences for binding the Src homology 3 (SH3) domains. To determine whether the proline-rich sequences interact with SH3 domains in several proteins, binding of recombinant SH3 domains to the meltrin α cytoplasmic domain was analysed by pull-down assays. The SH3 domains of Src and Yes bound strongly, but that of Abl or phosphatidylinositol 3-kinase p85 subunit did not. Full-length Grb2/Ash bound strongly, whereas its N-terminal SH3 domain alone did less strongly. Src and Grb2 in bovine brain extracts also bound to meltrin α cytoplasmic domain on affinity resin. Furthermore, immunoprecipitation with a monoclonal antibody to meltrin α resulted in coprecipitation of Src and Grb2 with meltrin α in cell extracts, suggesting that Src and Grb2 are associated in vivo with meltrin α cytoplasmic domain. This notion was also supported by the findings that exogenously expressed meltrin α cytoplasmic domain coexisted with Src and Grb2 on the membrane ruffles. The C-terminal Tyr901 of meltrin α was phosphorylated both in vitro and in cultured cells by v-Src. These results may imply that meltrin α cytoplasmic domain is involved in a signal transduction for some biological function through the interaction with SH3-containing proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Alexandropoulos K, Cheng G and Baltimore D. . 1995 Proc. Natl. Acad. Sci. USA 92: 3110–3114.
Alfandari D, Wolfsberg TG, White JM and DeSimone DW. . 1997 Dev. Biol. 182: 314–330.
Almeida EAC, Huovila A-PJ, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG and White JM. . 1995 Cell 81: 1095–1104.
Bader D, Masaki T and Fischman DA. . 1982 J. Cell. Biol. 95: 763–770.
Bar-Sagi D, Rotin D, Batzer A, Mandiyan V and Schlessinger J. . 1993 Cell 74: 83–91.
Ben-Ze'ev A. . 1997 Curr. Opin. Cell. Biol. 9: 99–108.
Birge RB, Knudsen BS, Besser D and Hanafusa H. . 1996 Genes Cells 1: 595–613.
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ and Cerretti DP. . 1997 Nature 385: 729–733.
Black RA and White JM. . 1998 Curr. Opin. Cell. Biol. 10: 654–659.
Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P and White JM. . 1992 Nature 356: 248–252.
Brown MT and Cooper JA. . 1996 Biochim. Biophys. Acta 1287: 121–149.
Carter N. . 1997 Current Protocols in Protein Science. Coligan JE, Dunn BM, Ploegh HL, Speicher DW and Wingfield PT (eds). John Wiley & Sons, Inc. pp. 13.7.1–13.7.22.
Chen MS, Almeida EAC, Huovila A-PJ, Takahashi Y, Shaw LM, Mercurio AM and White JM. . 1999 J. Cell. Biol. 144: 549–561.
Cho C, Bunch DO, Faure J-E, Goulding EH, Eddy EM, Primakoff P and Myles DG. . 1998 Science 281: 1857–1859.
Cohen GB, Ren R and Baltimore D. . 1995 Cell 80: 237–248.
Cross FR and Hanafusa H. . 1983 Cell 34: 597–607.
David-Pfeuty T and Nouvian-Dooghe Y. . 1990 J. Cell. Biol. 111: 3097–3116.
den Hertog J and Hunter T. . 1996 EMBO J. 15: 3016–3027.
Endo T. . 1992 J. Biochem. 112: 321–329.
Endo T and Nadal-Ginard B. . 1987 Cell 49: 515–526.
Endo T and Nadal-Ginard B. . 1998 J. Cell. Sci. 111: 1081–1093.
Endo T, Matsumoto K, Hama T, Ohtsuka Y, Katsura G and Obinata T. . 1996 J. Biol. Chem. 271: 27855–27862.
Feng S, Chen JK, Yu H, Simon JA and Schreiber SL. . 1994 Science 266: 1241–1247.
Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, Campbell ID and Waterfield MD. . 1993 Cell 75: 25–36.
Howard L, Nelson KK, Maciewicz RA and Blobel CP. . 1999 J. Biol. Chem. 274: 31693–31699.
Huovila A-PJ, Almeida EAC and White JM. . 1996 Curr. Opin. Cell. Biol. 8: 692–699.
Iba K, Albrechtsen R, Gilpin BJ, Loechel F and Wewer UM. . 1999 Am. J. Pathol. 154: 1489–1501.
Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umeta T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S and Mekada E. . 1998 EMBO J. 17: 7260–7272.
Kanemitsu MY, Loo LWM, Simon S, Lau AF and Eckhart W. . 1997 J. Biol. Chem. 272: 22824–22831.
Kurisaki T, Masuda A, Osumi N, Nabeshima Y and Fujisawa-Sehara A. . 1998 Mech. Dev. 73: 211–215.
Lim WA, Richards FM and Fox RO. . 1994 Nature 372: 375–379.
Loechel F, Gilpin BJ, Engvall E, Albrechtsen R and Wewer UM. . 1998 J. Biol. Chem. 273: 16993–16997.
Lum L, Reid MS and Blobel CP. . 1998 J. Biol. Chem. 273: 26236–26247.
Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A and Kaibuchi K. . 1996 EMBO J. 15: 2208–2216.
Matsumoto K, Asano T and Endo T. . 1997 Oncogene 15: 2409–2417.
Matuoka K, Shibata M, Yamakawa A and Takenawa T. . 1992 Proc. Natl. Acad. Sci. USA 89: 9015–9019.
Matuoka K, Shibasaki F, Shibata M and Takenawa T. . 1993 EMBO J. 12: 3467–3473.
Miki H, Miura K, Matuoka K, Nakata T, Hirokawa N, Orita S, Kaibuchi K, Takai Y and Takenawa T. . 1994 J. Biol. Chem. 269: 5489–5492.
Moss ML, Jin S-LC, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen W-J, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su J-L, Warner J, Willard D and Becherer JD. . 1997 Nature 385: 733–736.
Nath D, Slocombe PM, Stephens PE, Warn A, Hutchinson GR, Yamada KM, Docherty AJP and Murphy G. . 1999 J. Cell. Sci. 112: 579–587.
Nigg EA, Sefton BM, Hunter T, Walter G and Singer SJ. . 1982 Proc. Natl. Acad. Sci. USA 79: 5322–5326.
Okkenhaug K and Rottapel R. . 1998 J. Biol. Chem. 273: 21194–21202.
Pawson T. . 1995 Nature 373: 573–580.
Qi H, Rand MD, Wu X, Sestan N, Wang W, Rakic P, Xu T and Artavanis-Tsakonas S. . 1999 Science 283: 91–94.
Reznikoff CA, Brankow DW and Heidelberger C. . 1973 Cancer Res. 33: 3231–3238.
Roghani M, Becherer JD, Moss ML, Atherton RE, Erdjument-Bromage H, Arribas J, Blackburn RK, Weskamp G, Tempst P and Blobel CP. . 1999 J. Biol. Chem. 274: 3531–3540.
Sabe H, Knudsen B, Okada M, Nada S, Nakagawa H and Hanafusa H. . 1992 Proc. Natl. Acad. Sci. USA 89: 2190–2194.
Schlöndorff J and Blobel CP. . 1999 J. Cell. Sci. 112: 3603–3617.
Schwartz MA, Schaller MD and Ginsberg MH. . 1995 Annu. Rev. Cell. Dev. Biol. 11: 549–599.
Shibasaki F, Fukami K, Fukui Y and Takanawa T. . 1994 Biochem. J. 302: 551–557.
Sparks AB, Rider JE, Hoffman NG, Fowlkes DM, Quilliam LA and Kay BK. . 1996 Proc. Natl. Acad. Sci. USA 93: 1540–1544.
Thomas SM and Brugge JS. . 1997 Annu. Rev. Cell. Dev. Biol. 13: 513–609.
Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T and Tsukita S. . 1991 J. Cell. Biol. 113: 867–879.
Weskamp G, Krätzschmar J, Reid MS and Blobel CP. . 1996 J. Cell. Biol. 132: 717–726.
Wolfsberg TG, Primakoff P, Myles DG and White JM. . 1995 J. Cell. Biol. 131: 275–278.
Wolfsberg TG and White JM. . 1996 Dev. Biol. 180: 389–401.
Yaffe D and Saxel O. . 1977 Nature 270: 725–727.
Yang B, Jung D, Motto D, Meyer J, Koretzky G and Campbell KP. . 1995 J. Biol. Chem. 270: 11711–11714.
Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y and Fujisawa-Sehara A. . 1995 Nature 377: 652–656.
Yamada KM and Geiger B. . 1997 Curr. Opin. Cell. Biol. 9: 76–85.
Zhang X-P, Kamata T, Yokoyama K, Puzon-McLaughlin W and Takada Y. . 1998 J. Biol. Chem. 273: 7345–7350.
Acknowledgements
We are grateful to Dr Marius Sudol for the generous gift of the SH3 domain cDNA collections and to Drs Michinari Hamaguchi and Hidesaburo Hanafusa for pSR-XD2. C3H/10T1/2 cells were obtained from Japanese Cancer Research Resources Bank (JCRB). This study was partly supported by research grants from the Ministry of Education, Science, Sports, and Culture of Japan and from the Ministry of Health and Welfare of Japan for Nervous and Mental Disorders (8A-1 and 11B-1).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suzuki, A., Kadota, N., Hara, T. et al. Meltrin α cytoplasmic domain interacts with SH3 domains of Src and Grb2 and is phosphorylated by v-Src. Oncogene 19, 5842–5850 (2000). https://doi.org/10.1038/sj.onc.1203986
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203986
Keywords
This article is cited by
-
Adam12 plays a role during uterine decidualization in mice
Cell and Tissue Research (2009)
-
Evidence against roles for phorbol binding protein Munc13-1, ADAM adaptor Eve-1, or vesicle trafficking phosphoproteins Munc18 or NSF as phospho-state-sensitive modulators of phorbol/PKC-activated Alzheimer APP ectodomain shedding
Molecular Neurodegeneration (2007)
-
Identification of preferred protein interactions by phage‐display of the human Src homology‐3 proteome
EMBO reports (2006)
-
Epigenetic silencing of the adhesion molecule ADAM23 is highly frequent in breast tumors
Oncogene (2004)