Abstract
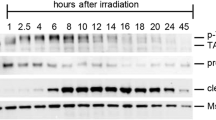
We studied the ability of F9 teratocarcinoma cells to arrest in G1/S and G2/M checkpoints after γ-irradiation. Wild-type p53 protein was rapidly accumulated in F9 cells after γ-irradiation, however, this was followed not by a G1/S arrest but by a short and reversible delay of the cell cycle in G2/M. In order to elucidate the reasons of the lack of G1/S arrest in F9 cells, we investigated the expression of p53 downstream target Cdk inhibitor p21WAF1/CIP1. In spite of p53-dependent activation of p21WAF1/CIP1 gene promoter and p21WAF1/CIP1 mRNA accumulation upon irradiation, the p21WAF1/CIP1 protein was not detected by either immunoblot or immunofluorescence techniques. However, the cells treated with a specific proteasome inhibitor lactacystin revealed the p21WAF1/CIP1 protein both in non-irradiated and irradiated cells. Therefore we suggest thatp21WAF1/CIP1 protein is degraded by a proteasome-dependent mechanism in F9 cells and the lack of G1/S arrest after γ-irradiation is due to this degradation. We also examined the expression and activity of cell cycle regulatory proteins: G1- and G2-cyclins and cyclin-dependent kinases. In the absence of functionalp21WAF1/CIP1 inhibitor, the activity of G1 cyclin/Cdk complexes was insufficiently inhibited to cause a G1 arrest, whereas a decrease of cdc2 and cyclin B1-associated kinase activities was enough to contribute to a reversible G2 arrest following γ-irradiation. Afterγ-irradiation, the majority of F9 cells undergo apoptosis implying that wt-p53 likely triggers pro-apoptotic gene expression in DNA damaged cells. Elimination of defected cells might ensure maintenance of genome integrity in the remaining cell population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Agapova LS, Ivanov AV, Sablina AA, Kopnin PB, Sokova OI, Chumakov PM and Kopnin BP. . 1999 Oncogene 18: 3135–3142.
Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R and Wahl GM. . 1998 Curr. Biol. 8: 145–155.
Atencia R, Garcia-Sanz M, Unda F and Arechaga J. . 1994 Exp. Cell Res. 214: 663–667.
Bates S, Ryan KM, Phillips A and Vousden KH. . 1998 Oncogene 17: 1691–1703.
Blagosklonny MV, Wu GS, Omura S and El-Deiry WS. . 1996 Biochem. Biophys. Res. Communs. 227: 564–569.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T and Hannon GJ. . 1995 Nature 377: 552–557.
Bulavin DV, Tararova ND, Aksenov ND, Pospelov VA and Pospelova TV. . 1999 Oncogene 18: 5611–5619.
Bunz F, Ditriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW and Vogelstein B. . 1998 Science 282: 1497–1500.
Carder P, Wyllie AH, Purdie SA, Morris RG, White S, Piris J and Bird CC. . 1993 Oncogene 3: 1397–1401.
Cayrol C and Ducommun B. . 1998 Oncogene 17: 2437–2444.
Cross SM, Sanchez SA, Morgan CA, Schimke MK, Ramel S, Idzeda RL, Raskind WH and Reid BJ. . 1995 Science 267: 1353–1356.
Deng C, Zhang P, Harper JW, Elledge SJ and Leder P. . 1995 Cell 82:: 675–684.
Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK and Dotto GP. . 1998 Science 280: 1069–1072.
Dulic V, Stein GH, Far DF and Reed SI. . 1998 Mol. Cell. Biol. 18: 546–557.
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW and Vogelstein B. . 1993 Cell 75: 817–825.
Elledge SJ. . 1996 Science 274: 1664–1672.
Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ and Schreiber SL. . 1995 Science 268: 726–731.
Glotzer M, Murray AW and Kirscher MW. . 1991 Nature 349: 132–138.
Hollstein M, Sidransky D, Vogelstein B and Harris C. . 1991 Science 253: 49–53.
Jimenez GS, Khan SH, Stommel JM and Wahl JM. . 1999 Oncogene 18: 7656–7665.
Khan SH and Wahl GM. . 1998 Cancer Res. 58: 396–401.
Koff A, Cross F, Fisher A, Schumacher J, Leguellee K, Philippe M and Roberts JM. . 1991 Cell 66: 1217.
Kubbutat MHG and Vousden KH. . 1998 Mol. Med. Today 4: 250–256.
Kuerbitz SJ, Plunkett BS, Walsh WV and Kastan MB. . 1992 Proc. Natl. Acad. Sci. USA 89: 7491–7495.
Langley RE, Palagoor ST, Coleman CN and Bump EA. . 1993 Radiat. Res. 136: 320–326.
Langley RE, Quartuccio SG, Keannealey PT, Coleman CN and Bump EA. . 1995 Radiat. Res. 144: 90–96.
Lanni JS and Jacks T. . 1998 Molec. Cell. Biol. 18: 1055–1064.
Levine AJ. . 1997 Cell 88: 323–331.
Lutzker SG and Levine AJ. . 1996 Nature Med. 2: 804–810.
Malashicheva AB, Kislyakova TV and Pospelov VA. . 1998 Tsytologia (Russ.) 40: 14–22.
Mayo LD and Berberich S. . 1996 Oncogene 13: 2315–2321.
Medema RH, Klompmaker R, Smits VA and Rijksen G. . 1998 Oncogene 16: 431–441.
Orlovskaya EI, Kislyakova TV, Osipov KA, Malashicheva AB and Pospelov VA. . 1997 Molec. Biol. (Mosk). 31: 224–231.
Pagano M, Tam SW, Theodoras AM, Beer Romero P, Del Sal G, Chau V, Yew PR, Draetta GF and Rolfe M. . 1995 Science 269: 682–685.
Pennica D, Goeddel DV, Hayflick JS, Reich NC, Anderson CW and Levine AJ. . 1984 Virology 134: 477–482.
Perreault J and Lemieux R. . 1993 J. Cell. Physiol. 156: 186–293.
Poon RYC and Hunter T. . 1998 Oncogene 16: 1333–1343.
Savatier P, Huang S, Szekely L, Wiman KG and Samarut J. . 1994 Oncogene 9: 2261–2268.
Savatier P, Lapillonne H, van GL, Rudkin BB and Samarut J. . 1996 Oncogene 12: 309–322.
Sleigh MJ. . 1992 BioEssays 14: 769–775.
Stewart ZA, Leash SD and Pietenpol J. . 1999 Mol. Cell. Biol. 19: 205–215.
Strickland S, Smith KK and Marotty KR. . 1980 Cell 21: 347–355.
Waldman T, Lengauer C, Kinzler KW and Vogelstein B. . 1996 Nature 381: 713–716.
Acknowledgements
We are grateful to Dr Yu M Rosanov for assistance in flow cytometry experiments, we thank Dr W El-Deiry who kindly provided WWP-luc plasmid and also Dr A Zantema for anti-cdk2 antibodies. This work was supported by Russian Foundation of Basic Research grants 00-04-49389 and INTAS 99-1036 (VAP), 00-04-49024 (TVK) and NCI-NIH CDA-NIS (TVK), and by the grant for young scientists of St Petersburg's mayor ASP no. 298414 (ABM).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Malashicheva, A., Kislyakova, T., Aksenov, N. et al. F9 embryonal carcinoma cells fail to stop at G1/S boundary of the cell cycle after γ-irradiation due to p21WAF1/CIP1 degradation. Oncogene 19, 3858–3865 (2000). https://doi.org/10.1038/sj.onc.1203736
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203736
Keywords
This article is cited by
-
p21 provides stage specific DNA damage control to preimplantation embryos
Oncogene (2007)
-
Low p21Waf1/Cip1 protein level sensitizes testicular germ cell tumor cells to Fas-mediated apoptosis
Oncogene (2004)
-
Downregulation of c-fos gene transcription in cells transformed by E1A and cHa-ras oncogenes: a role of sustained activation of MAP/ERK kinase cascade and of inactive chromatin structure at c-fos promoter
Oncogene (2002)