Abstract
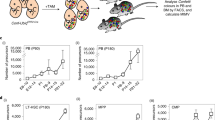
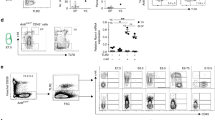
The c-Myb transcriptional regulator is crucial to the development and functioning of haemopoietic cells, so much so that mouse embryos homozygous for an inactivated c-myb allele die from anaemia at about day 15 of gestation. By analysing c-myb−/− chimaeras we show that no mature cells of any lymphoid or myeloid lineage can be detected in adult haemopoietic tissues. This demonstrates that the effects of c-myb ablation on haemopoiesis are cell autonomous and correlates with an absence in the c-myb−/− foetal liver of uni- and multi-lineage CFUs. Indeed, CFU assays performed on E8.5 yolk sac cells revealed that haemopoietic progenitors are already defective at this stage. However, although cells expressing high levels of c-Kit were absent, we could detect a high proportion of CD34+CD45+ cells in the c-myb−/− foetal liver. Examination of chimaeric embryos revealed that c-myb−/− donor-derived CD34+/Kit+ cells, representing committed definitive progenitors, initially populated the foetal liver, but are unable to expand like wild type progenitors. Our results showing no megakaryocytic CFUs and a reduction in the absolute numbers of megakaryocytes in the c-myb−/− foetal liver also refute early suggestions that megakaryopoiesis is unaffected by the absence of c-Myb.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Allen RD, Bender TP and Siu G. . 1999 Genes Dev. 13: 1073–1078.
Bernex F, de Sepulveda P, Kress C, Elbaz C, Delouis C and Panthier J-J. . 1996 Development 122: 3023–3033.
Bonifer C, Faust N, Geiger H and Müller AM. . 1998 Immunology Today 19: 236–241.
Clarke D, Vegiopoulos A, Crawford A, Mucenski M, Bonifer C and Frampton J. . 2000 Oncogene this issue.
Dzierzak E, Medvinsky A and de Bruijn M. . 1998 Immunol. Today 19: 228–236.
Frampton J, Ramqvist T and Graf T. . 1996 Genes & Dev. 10: 2720–2731.
Frampton J and Graf T. . 1998 In Transcription Factors in Eukaryotes, Papavassiliou AG (ed.). RG Landes Co: Georgetown pp. 287–295.
Hogan B, Beddington R, Constantini F and Lacy E. . 1994 Manipulating the mouse embryo: A laboratory manual (2nd edn). Cold Spring Harbor Laboratory Press, New York.
Hogg A, Schirm S, Nakagoshi H, Bartley P, Ishii S, Bishop JM and Gonda TJ. . 1997 Oncogene 15: 2885–2898.
Krause DS, Mucenski ML, Lawler AM and May WS. . 1998 Exp. Hematol. 26: 1086–1092.
Ledbetter JA, Goding JW, Tsu TT and Herzenberg LA. . 1979 Immunogenetics 8: 347–360.
Lin HH, Sternfeld DC, Shinpock SG, Popp RA and Mucenski ML. . 1996 Curr. Topic. Microbiol. Immun. 211: 79–87.
Lin HH, Stubbs LJ and Mucenski ML. . 1997 Genomics 41: 301–308.
Lipsick JS and Wang D-M. . 1999 Oncogene 18: 3047–3055.
Matsumara G and Sasaki K. . 1989 J. Anat. 167: 181–187.
Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AAT and Seidman JG. . 1992 Mol. Cell. Biol. 12: 2391–2395.
Mucenski ML, McLain K, Kier AB, Swederlow SH, Schreiner CM, Miller TA, Pietryga DW, Scott Jr WJ and Potter SS. . 1991 Cell 65: 677–689.
Mukouyama Y-S, Chiba N, Mucenski ML, Satake M, Miyajima A, Hara T and Watanabe T. . 1999 Curr. Biol. 9: 833–836.
Ness SA. . 1996 Biochim. Biophys. Acta. 1288: F123–F139.
Ogawa M, Nishikawa S, Yoshinaga K, Hayashi S-I, Kunisada T, Nakao J, Kina T, Sudo T, Kodama H and Nishikawa S-I. . 1993 Development 117: 1089–1098.
Oh I-H and Reddy EP. . 1999 Oncogene 18: 3017–3033.
Okuda T, van Deursen J, Hiebert SW, Grosveld G and Downing JR. . 1996 Cell 84: 321–330.
Ratajczak MZ, Perrotti D, Melotti P, Powzaniuk M, Calabretta B, Onodera K, Kregenow DA, Machalinski B and Gewirtz AM. . 1998 Blood 91: 1934–1946.
Taylor D, Badiani P and Weston K. . 1996 Genes Dev. 10: 2732–2744.
Tsai F-Y, Keller G, Kuo FC, Weiss MJ, Chen J-Z, Rosenblatt M, Alt FW and Orkin SH. . 1994 Nature 371: 221–226.
Tsai F-Y and Orkin SH. . 1997 Blood 89: 3636–3643.
Weiss MJ, Keller G and Orkin SH. . 1994 Genes Dev. 8: 1184–1197.
Weston K. . 1998 Curr. Opin. Genet. Dev. 8: 76–81.
Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM and Orlic D. . 1997 Immunity 7: 335–344.
Acknowledgements
We thank Amin Rahmentulla and Colin Hetherington for help in setting up the chimaera experiments. We are grateful to Toshio Watanabe for his considerable assistance in enabling us to obtain the c-myb knock out mice. We would also like to thank Constanze Bonifer, Nikla Emambokus, Thomas Graf, Andrew Thomson and Bill Wood for critical reviewing of the manuscript and all other members of the Frampton lab for discussions and advice. This work was supported by the Wellcome Trust as part of a Senior Fellowship award to J Frampton.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sumner, R., Crawford, A., Mucenski, M. et al. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene 19, 3335–3342 (2000). https://doi.org/10.1038/sj.onc.1203660
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203660
Keywords
This article is cited by
-
Physiology and diseases of tissue-resident macrophages
Nature (2023)
-
Reversal of MYB-dependent suppression of MAFB expression overrides leukaemia phenotype in MLL-rearranged AML
Cell Death & Disease (2023)
-
Identification of a c-MYB-directed therapeutic for acute myeloid leukemia
Leukemia (2022)
-
Lyl-1 regulates primitive macrophages and microglia development
Communications Biology (2021)
-
Targeting acute myeloid leukemia by drug-induced c-MYB degradation
Leukemia (2018)