Abstract
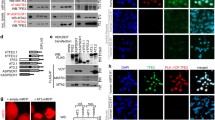
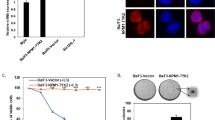
The papillary renal cell carcinoma-associated t(X;1)(p11;q21) leads to fusion of the transcription factor TFE3 gene on the X-chromosome to a novel gene, PRCC, on chromosome 1. As a result, two putative fusion proteins are formed: PRCCTFE3, which contains all known domains for DNA binding, dimerization, and transactivation of the TFE3 protein, and the reciprocal product TFE3PRCC. Upon transfection into COS cells, both wild type and fusion proteins were found to be located in the nucleus. When comparing the transactivating capacities of these (fusion) proteins, significant differences were noted. PRCCTFE3 acted as a threefold better transactivator than wild type TFE3 both in a TFE3-specific and in a general (Zebra) reporter assay. In addition, PRCC and the two fusion proteins were found to be potent transactivators in the Zebra reporter assay. We propose that, as a result of the (X;1) translocation, fusion of the N-terminal PRCC sequences to TFE3 alters the transactivation capacity of the transcription factor thus leading to aberrant gene regulation and, ultimately, tumor formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Artandi SE, Merrell K, Avitahl N, Wong KK and Calame K . 1995 Nucleic Acids Res 23: 3865–3871.
Askovic S and Baumann R . 1997 BioTechniques 22: 948–951.
Banerji J, Olson L and Schaffner W . 1983 Cell 33: 729–740.
Beckmann H, Su LK and Kadesch T . 1990 Genes Dev 4: 167–179.
Beckmann H and Kadesch T . 1991 Genes Dev 5: 1057–1066.
Braun BS, Frieden R, Lessnick SL, May WA and Denny CT . 1995 Mol Cell Biol 15: 4623–4630.
Clark J, Lu Y-L, Sidhar SK, Parker C, Gill S, Smedley D, Hamoudi R, Linehan WM, Shipley J and Cooper CS . 1997 Oncogene 15: 2233–2239.
de Jong B, Molenaar IM, Leeuw JA, Idenburg VIS, and Oosterhuis JW . 1986 Cancer Genet Cytogenet 21: 165–169.
Delattre O, Zucman J, Ploustagel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, Aurias A and Thomas G . 1992 Nature 359: 162–165.
Dijkhuizen T, van den Berg E, Stoerkel S, Terpe H-J, Buerger H and de Jong B . 1998 Cancer Genet Cytogenet 104: 74–76.
Edlund T, Walker MD, Barr PJ and Rutter WJ . 1985 Science 230: 912–916.
Gerber H-P, Seipel K, Georgiev O, Hoefferer M, Hug M, Rusconi S and Schaffner W . 1994 Science 263: 808–811.
Gillies SD, Morrison SL, Oi VT and Tonegawa S . 1983 Cell 33: 717–728.
Hua XX, Liu XD, Ansari DO and Lodish HF . 1998 Genes Dev 12: 3084–3095.
Meloni AM, Dobbs RMJ, Pontes JE and Sandberg AA . 1993 Cancer Genet Cytogenet 65: 1–6.
Neuberger MS . 1983 EMBO J 2: 1373–1378.
Rabbitts TH . 1994 Nature 372: 143–149.
Rao E, Dang W, Tian G and Sen RJ . 1997 J Biol Chem 272: 6722–6732.
Ren R, Mayer BJ, Cicchetti P and Baltimore D . 1993 Science 259: 1157–1161.
Rogaia D, Grignani F, Carbone R, Riganelli D, Lococo F, Nakamura T, Croce CM, Difiore PP and Pelicci PG . 1997 Cancer Res 57: 799–802.
Roman C, Cohn L and Calame K . 1991 Science 254: 94–97.
Roman C, Matera AG, Cooper C, Artandi S, Blain S, Ward DC and Calame K . 1992 Mol Cell Biol 12: 817–827.
Sidhar SK, Clark J, Gill S, Hamoundi R, Crew AJ, Gwilliam R, Ross M, Linehan WM, Birdsall S, Shipley J and Cooper CS . 1996 Hum Mol Genet 5: 1333–1338.
Sorensen PHB and Triche TJ . 1996 Semin Cancer Biol 7: 3–14.
Sudol M . 1998 Oncogene 17: 1469–1474.
Takebayashi K, Chida K, Tsukamoto I, Morii E, Munakata H, Arnheiter H, Kuroki T, Kitamura Y and Nomura S . 1996 Mol Cell Biol 16: 1203–1211.
Tonk V, Wilson KS, Timmons CF, Schneider NR and Tomlinson GE . 1995 Cancer Genet Cytogenet 81: 72–75.
Towbin H, Staehelin T and Gordon J . 1979 Proc Natl Acad Sci USA 76: 4350–4354.
Weis K . 1998 Trends Biochem Sci 23: 185–189.
Weterman MAJ, Wilbrink M, Janssen I, Janssen HAP, van den Berg E, Fisher SE, Craig I and Geurts van Kessel A . 1996a Cytogenet Cell Genet 75: 2–6.
Weterman MAJ, Wilbrink M and Geurts van Kessel A . 1996b Proc Natl Acad Sci USA 93: 15294–15298.
Acknowledgements
The TFE3 cDNA expression constructs (TFE3-L, TFE3-S), and μE3 reporter constructs were generously provided by Dr Calame. The Zebra and Zd constructs were a kind gift of Dr Askovic. This work was supported by the Dutch Cancer Society, grant 98-1804.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weterman, M., van Groningen, J., Jansen, A. et al. Nuclear localization and transactivating capacities of the papillary renal cell carcinoma-associated TFE3 and PRCC (fusion) proteins. Oncogene 19, 69–74 (2000). https://doi.org/10.1038/sj.onc.1203255
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203255
Keywords
This article is cited by
-
A new glance at autophagolysosomal-dependent or -independent function of transcriptional factor EB in human cancer
Acta Pharmacologica Sinica (2023)
-
High-throughput and targeted drug screens identify pharmacological candidates against MiT-translocation renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Nuclear translocation of ASPL-TFE3 fusion protein creates favorable metabolism by mediating autophagy in translocation renal cell carcinoma
Oncogene (2021)
-
TFE3 fusions escape from controlling of mTOR signaling pathway and accumulate in the nucleus promoting genes expression in Xp11.2 translocation renal cell carcinomas
Journal of Experimental & Clinical Cancer Research (2019)
-
Translation control during prolonged mTORC1 inhibition mediated by 4E-BP3
Nature Communications (2016)