Abstract
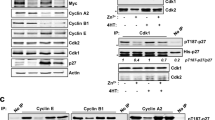
The G1-S transition in mammalian cells has been demonstrated to require the cyclin-dependent kinases cdk2, cdk3 and cdk4/6. Here we show that a novel kinase activity associated with cdk3 fluctuates throughout the cell cycle differently from the expression of cyclin D1-, E- and A-associated kinase activities. Cdk3 kinase activity is neither affected by p16 (in contrast to cdk4/6) nor by E2F-1 (in contrast to cdk2), but is downregulated upon transient p27 expression. We found cdk3 to bind to p21 and p27. We provide evidence that p27 could be involved in the regulation of the cell cycle fluctuation of cdk3 activity: cdk3 protein does not fluctuate and interaction of cdk3 with p27, but not with p21, is lost when cdk3 kinase becomes active during the cell cycle. In Myc-overexpressing cells, but not in normal Rat1 cells, constitutive ectopic expression of cdk3 induces specific upregulation of cdk3-associated kinase activity that is still cell cycle phase dependent. Ectopic cdk3, but not cdk2, enhances Myc-induced proliferation and anchorage-independent growth associated with Myc activation, without effects on cyclin D1, E and A protein expression or kinase activities. High levels of cdk3 in Myc-overexpressing cells trigger up- and deregulation of E2F-dependent transcription without inducing the E2F-DNA binding capacity. In contrast to all other studied positive G1 regulators, cdk3 is unable to cooperate with ras in fibroblast transformation suggesting a function of cdk3 in G1 progression that is different from cyclin D- or E-associated kinase activities. Our data provide first insights into the regulation of cdk3-associated kinase activity and suggest a model how cdk3 participates in the regulation of the G1-S transition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Braun, K., Hölzl, G., Soucek, T. et al. Investigation of the cell cycle regulation of cdk3-associated kinase activity and the role of cdk3 in proliferation and transformation. Oncogene 17, 2259–2269 (1998). https://doi.org/10.1038/sj.onc.1202145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1202145
Keywords
This article is cited by
-
New insights into phenotypic heterogeneity for the distinct lipid accumulation of Schizochytrium sp. H016
Biotechnology for Biofuels and Bioproducts (2022)
-
Phosphorylation of NFAT3 by CDK3 induces cell transformation and promotes tumor growth in skin cancer
Oncogene (2017)
-
Implication of human N-α-acetyltransferase 5 in cellular proliferation and carcinogenesis
Oncogene (2008)
-
Enhancement of cell proliferation in various mammalian cell lines by gene insertion of a cyclin-dependent kinase homolog
BMC Biotechnology (2007)
-
Regulation of the G1 phase of the mammalian cell cycle
Cell Research (2000)