Abstract
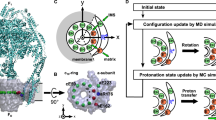
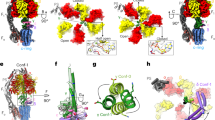
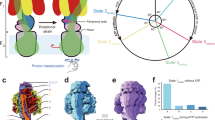
The mitochondrial membrane protein FoF1-ATP synthase synthesizes adenosine triphosphate (ATP), the universal currency of energy in the cell. This process involves mechanochemical energy transfer from a rotating asymmetric γ-'stalk' to the three active sites of the F1 unit, which drives the bound ATP out of the binding pocket. Here, the primary structural changes associated with this energy transfer in F1-ATP synthase were studied with multi-nanosecond molecular dynamics simulations. By forced rotation of the γ-stalk that mimics the effect of proton motive Fo-rotation during ATP synthesis, a time-resolved atomic model for the structural changes in the F1 part in terms of propagating conformational motions is obtained. For these, different time scales are found, which allows the separation of nanosecond from microsecond conformational motions. In the simulations, rotation of the γ-stalk lowers the ATP affinity of the βTP binding pocket and triggers fast, spontaneous closure of the empty βE subunit. The simulations explain several mutation studies and the reduced hydrolysis rate of γ-depleted F1-ATPase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mitchell, P. Nature 191, 144–148 (1961).
Boyer, P.D. Biochim. Biophys. Acta 1140, 215–250 (1993).
Duncan, T.M., Bulygin, V.V., Zhou, Y., Hutcheon, M.L. & Cross, R.L. Proc. Natl. Acad. Sci. USA 92, 10964–10968 (1995).
Sabbert, D., Engelbrecht, S. & Junge, W. Nature 381, 623–625 (1996).
Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. Nature 386, 299–302 (1997).
Abrahams, J.P., Leslie, A.G.W., Lutter, L. & Walker, J.E. Nature 370, 621–628 (1994).
Gibbons, C., Montgomery, M.G., Leslie, A.G.W. & Walker, J.E. Nature Struct. Biol. 7, 1055–1061 (2000).
Weber, J. & Senior, A.E. Biochim. Biophys. Acta 1319, 19–58 (1997).
Pänke, O. & Rumberg, B. Biochim. Biophys. Acta 1322, 183–194 (1997).
Ren, G. & Allison, W.S. Biochim. Biophys. Acta 1458, 221–233 (2000).
Hara, K.Y. et al. J. Biol. Chem. 275, 14260–14263 (2000).
Boyer, P.D. Annu. Rev. Biochem. 66, 717–749 (1997).
Engelbrecht, S. & Junge, W. FEBS Lett. 414, 485–491 (1997).
Cherepanov, D.A., Mulkidjanian, A.Y. & Junge, W. FEBS Lett. 449, 1–6 (1999).
Weber, J., Nadanaciva, S. & Senior, A.E. FEBS Lett. 483, 1–5 (2000).
Oster, G. & Wang, H.Y. Biochim. Biophys. Acta 1458, 482–510 (2000).
Grubmüller, H., Heymann, B. & Tavan, P. Science 271, 997–999 (1996).
Izrailev, S., Stepaniants, S., Balsera, M., Oono, Y. & Schulten, K. Biophys. J. 72, 1568–1581 (1997).
Dunn, S.D. & Futai, M. J. Biol. Chem. 255, 113–118 (1980).
Miwa, K. & Yoshida, M. Proc. Natl. Acad. Sci. USA 86, 6484–6487 (1989).
Yasuda, R., Noji, H., Yoshida, M., Kinosita, K. & Itoh, H. Nature 410, 898–904 (2001).
Pänke, O., Cherepanov, D.A., Gumbiowski, K., Engelbrecht, S. & Junge, W. Biophys. J. 81, 1220–1233 (2001).
Weber, J., Hammond, S.T., Wilke-Mounts, S. & Senior, A.E. Biochemistry 37, 608–614 (1998).
Menz, R.I., Walker, J.E. & Leslie, A.G.W. Cell 106, 331 (2001).
Heymann, B. & Grubmüller, H. Chem. Phys. Lett. 303, 1–9 (1999).
Nadanaciva, S., Weber, J., Wilke-Mounts, S. & Senior, A.E. Biochemistry 38, 15493–15499 (1999).
Le, N.P. et al. Biochemistry 39, 2778–2783 (2000).
Nadanaciva, S., Weber, J. & Senior, A.E. Biochemistry 38, 7670–7677 (1999).
Eichinger, M., Heller, H. & Grubmüller, H. In Workshop on molecular dynamics on parallel computers. (eds Esser, R. et al.) 154–174 (World Scientific, Singapore; 2000).
Brooks, B.R. et al. J. Comp. Chem. 4, 187–217 (1983).
Eichinger, M., Grubmüller, H., Heller, H. & Tavan, P. J. Comp. Chem. 18, 1729–1749 (1997).
Jorgensen, W.L., Chandrasekhar, J. & Madura, J.D. J. Chem. Phys. 79, 926–935 (1983).
Koradi, R., Billeter, M., & Wüthrich, K. J. Mol. Graphics 14, 51–55 (1996).
Sayle, R.A. & Milnerwhite, E.J. Trends Biochem. Sci. 20 374–376 (1995).
Acknowledgements
We thank W. Junge, A. Engel, B. de Groot, B. Heymann, K. Schulten, W. Allison, D. Chandler, V. Helms, M. Hofmann, K. Kinosita, V. Knecht, R. Lang, G. Oster, G. Schröder and H. Wang for stimulating discussions and for critical reading of the manuscript; B. de Groot for help with the GROMACS program package; and G. Schneider and O. Haan for their support. Computer time was provided by the Göttingen computer center (GWDG) and the Paderborn center for parallel computing (PC2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Böckmann, R., Grubmüller, H. Nanoseconds molecular dynamics simulation of primary mechanical energy transfer steps in F1-ATP synthase. Nat Struct Mol Biol 9, 198–202 (2002). https://doi.org/10.1038/nsb760
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb760
This article is cited by
-
The FOF1 ATP synthase: from atomistic three-dimensional structure to the rotary-chemical function
Photosynthesis Research (2017)